Rolipram-Loaded Polymeric Micelle Nanoparticle Reduces Secondary Injury after Rat Compression Spinal Cord Injury
- PMID: 29065765
- PMCID: PMC5793955
- DOI: 10.1089/neu.2017.5092
Rolipram-Loaded Polymeric Micelle Nanoparticle Reduces Secondary Injury after Rat Compression Spinal Cord Injury
Abstract
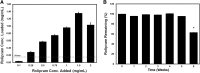
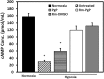
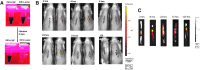
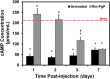
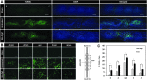
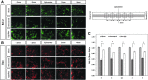
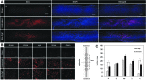
Among the complex pathophysiological events following spinal cord injury (SCI), one of the most important molecular level consequences is a dramatic reduction in neuronal cyclic adenosine monophosphate (cAMP) levels. Many studies shown that rolipram (Rm), a phosphodiesterase IV inhibitor, can protect against secondary cell death, reduce inflammatory cytokine levels and immune cell infiltration, and increase white matter sparing and functional improvement. Previously, we developed a polymeric micelle nanoparticle, poly(lactide-co-glycolide)-graft-polyethylenimine (PgP), for combinatorial delivery of therapeutic nucleic acids and drugs for SCI repair. In this study, we evaluated PgP as an Rm delivery carrier for SCI repair. Rolipram's water solubility was increased ∼6.8 times in the presence of PgP, indicating drug solubilization in the micelle hydrophobic core. Using hypoxia as an in vitro SCI model, Rm-loaded PgP (Rm-PgP) restored cAMP levels and increased neuronal cell survival of cerebellar granular neurons. The potential efficacy of Rm-PgP was evaluated in a rat compression SCI model. After intraspinal injection, 1,1'-dioctadecyl-3,3,3',3'-tetramethyl indotricarbocyanine Iodide-loaded PgP micelles were retained at the injection site for up to 5 days. Finally, we show that a single injection of Rm-PgP nanoparticles restored cAMP in the SCI lesion site and reduced apoptosis and the inflammatory response. These results suggest that PgP may offer an efficient and translational approach to delivering Rm as a neuroprotectant following SCI.
Keywords: cAMP; compression spinal cord injury; polymeric micelle nanoparticle; rolipram; secondary injury.
Conflict of interest statement
No competing financial interests exist.
Figures


Similar articles
-
Rolipram-loaded PgP nanoparticle reduces secondary injury and enhances motor function recovery in a rat moderate contusion SCI model.Nanomedicine. 2023 Sep;53:102702. doi: 10.1016/j.nano.2023.102702. Epub 2023 Aug 11. Nanomedicine. 2023. PMID: 37574117 Free PMC article.
-
Therapeutic efficacy of rolipram delivered by PgP nanocarrier on secondary injury and motor function in a rat TBI model.Nanomedicine (Lond). 2022 Mar;17(7):431-445. doi: 10.2217/nnm-2021-0271. Epub 2022 Feb 21. Nanomedicine (Lond). 2022. PMID: 35184609 Free PMC article.
-
The therapeutic profile of rolipram, PDE target and mechanism of action as a neuroprotectant following spinal cord injury.PLoS One. 2012;7(9):e43634. doi: 10.1371/journal.pone.0043634. Epub 2012 Sep 19. PLoS One. 2012. PMID: 23028463 Free PMC article.
-
Neurotrophic factors for spinal cord repair: Which, where, how and when to apply, and for what period of time?Brain Res. 2015 Sep 4;1619:36-71. doi: 10.1016/j.brainres.2014.10.049. Epub 2014 Nov 1. Brain Res. 2015. PMID: 25451132 Review.
-
Injectable Hydrogels for Spinal Cord Repair: A Focus on Swelling and Intraspinal Pressure.Cells Tissues Organs. 2016;202(1-2):67-84. doi: 10.1159/000446697. Epub 2016 Oct 5. Cells Tissues Organs. 2016. PMID: 27701162 Review.
Cited by
-
Silencing of Long Noncoding RNA Growth Arrest-Specific 5 Alleviates Neuronal Cell Apoptosis and Inflammatory Responses Through Sponging microRNA-93 to Repress PTEN Expression in Spinal Cord Injury.Front Cell Neurosci. 2021 May 14;15:646788. doi: 10.3389/fncel.2021.646788. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34054430 Free PMC article.
-
Targeted therapy and deep learning insights into microglia modulation for spinal cord injury.Mater Today Bio. 2024 Jun 8;27:101117. doi: 10.1016/j.mtbio.2024.101117. eCollection 2024 Aug. Mater Today Bio. 2024. PMID: 38975239 Free PMC article.
-
LncRNA DGCR5 suppresses neuronal apoptosis to improve acute spinal cord injury through targeting PRDM5.Cell Cycle. 2018;17(16):1992-2000. doi: 10.1080/15384101.2018.1509622. Epub 2018 Sep 11. Cell Cycle. 2018. PMID: 30146926 Free PMC article.
-
Suicide Gene Therapy By Amphiphilic Copolymer Nanocarrier for Spinal Cord Tumor.Nanomaterials (Basel). 2019 Apr 8;9(4):573. doi: 10.3390/nano9040573. Nanomaterials (Basel). 2019. PMID: 30965667 Free PMC article.
-
NPC transplantation rescues sci-driven cAMP/EPAC2 alterations, leading to neuroprotection and microglial modulation.Cell Mol Life Sci. 2022 Jul 29;79(8):455. doi: 10.1007/s00018-022-04494-w. Cell Mol Life Sci. 2022. PMID: 35904607 Free PMC article.
References
-
- Mothe A. and Tator C. (2013). Review of transplantation of neural stem/progenitor cells for spinal cord injury. Int. J. Dev. Neurosci. 31, 701–713 - PubMed
-
- Colello R., Chow W., Bigbee J., Lin C., Dalton D., Brown D., Jha B., Mathern B., Lee K., and Simpson D. (2016). The incorporation of growth factor and chondroitinase ABC into an electrospun scaffold to promote regrowth following spinal cord injury. J. Tissue Eng. Regen. Med. 10, 656–668 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

