The value of blood cytokines and chemokines in assessing COPD
- PMID: 29065892
- PMCID: PMC5655820
- DOI: 10.1186/s12931-017-0662-2
The value of blood cytokines and chemokines in assessing COPD
Abstract
Background: Blood biomarkers are increasingly used to stratify high risk chronic obstructive pulmonary disease (COPD) patients; however, there are fewer studies that have investigated multiple biomarkers and replicated in multiple large well-characterized cohorts of susceptible current and former smokers.
Methods: We used two MSD multiplex panels to measure 9 cytokines and chemokines in 2123 subjects from COPDGene and 1117 subjects from SPIROMICS. These biomarkers included: interleukin (IL)-2, IL-6, IL-8, IL-10, tumor necrosis factor (TNF)-α, interferon (IFN)-γ, eotaxin/CCL-11, eotaxin-3/CCL-26, and thymus and activation-regulated chemokine (TARC)/CCL-17. Regression models adjusted for clinical covariates were used to determine which biomarkers were associated with the following COPD phenotypes: airflow obstruction (forced expiratory flow at 1 s (FEV1%) and FEV1/forced vital capacity (FEV1/FVC), chronic bronchitis, COPD exacerbations, and emphysema. Biomarker-genotype associations were assessed by genome-wide association of single nucleotide polymorphisms (SNPs).
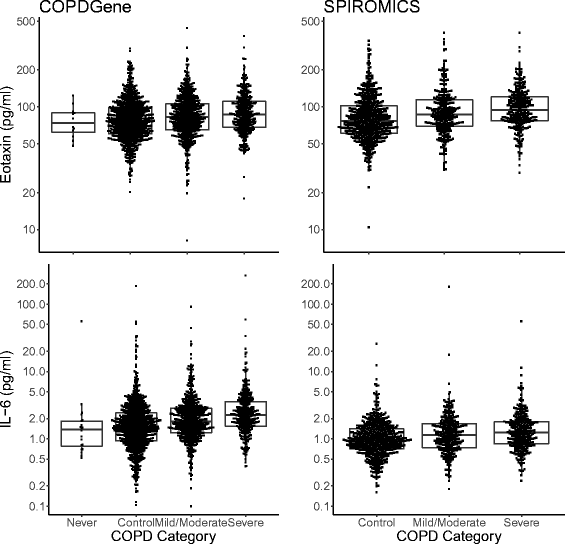
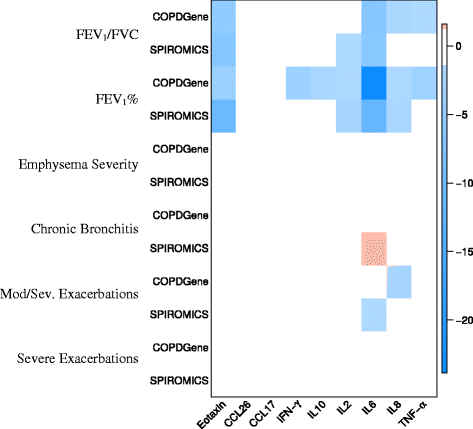
Results: Eotaxin and IL-6 were strongly associated with airflow obstruction and accounted for 3-5% of the measurement variance on top of clinical variables. IL-6 was associated with progressive airflow obstruction over 5 years and both IL-6 and IL-8 were associated with progressive emphysema over 5 years. None of the biomarkers were consistently associated with chronic bronchitis or COPD exacerbations. We identified one novel SNP (rs9302690 SNP) that was associated with CCL17 plasma measurements.
Conclusion: When assessing smoking related pulmonary disease, biomarkers of inflammation such as IL-2, IL-6, IL-8, and eotaxin may add additional modest predictive value on top of clinical variables alone.
Trial registration: COPDGene (ClinicalTrials.gov Identifier: NCT02445183 ). Subpopulations and Intermediate Outcomes Measures in COPD Study (SPIROMICS) ( ClinicalTrials.gov Identifier: NCT 01969344 ).
Trial registration: ClinicalTrials.gov NCT02445183 NCT01969344.
Conflict of interest statement
Ethics approval and consent to participate
The study protocols were approved by the local ethics committees listed below. Informed written consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- United States. Public Health Service. Office Of The Surgeon General . The health consequences of smoking--50 years of progress : a report of the surgeon general. Rockville: U.S. Department Of Health And Human Services, Public Health Service, Office Of The Surgeon General; 2014.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U01 HL089897/HL/NHLBI NIH HHS/United States
- R01 HL095432/HL/NHLBI NIH HHS/United States
- R01 HL126596/HL/NHLBI NIH HHS/United States
- U01 HL137880/HL/NHLBI NIH HHS/United States
- HHSN268200900014C/HL/NHLBI NIH HHS/United States
- R01 HL089897/HL/NHLBI NIH HHS/United States
- HHSN268200900019C/HL/NHLBI NIH HHS/United States
- K24 HL137013/HL/NHLBI NIH HHS/United States
- R01 HL089856/HL/NHLBI NIH HHS/United States
- U01 HL089856/HL/NHLBI NIH HHS/United States
- HHSN268200900015C/HL/NHLBI NIH HHS/United States
- R01 HL129937/HL/NHLBI NIH HHS/United States
- HHSN268200900016C/HL/NHLBI NIH HHS/United States
- HHSN268200900017C/HL/NHLBI NIH HHS/United States
- HHSN268200900020C/HL/NHLBI NIH HHS/United States
- HHSN268200900013C/HL/NHLBI NIH HHS/United States
- R01 HL124233/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

