Tissue transport affects how treatment scheduling increases the efficacy of chemotherapeutic drugs
- PMID: 29066114
- PMCID: PMC9909584
- DOI: 10.1016/j.jtbi.2017.10.022
Tissue transport affects how treatment scheduling increases the efficacy of chemotherapeutic drugs
Abstract
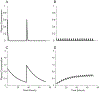
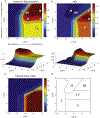
A method to predict the effect of tissue transport on the scheduling of chemotherapeutic treatment could increase efficacy. Many drugs with desirable pharmacokinetic properties fail in vivo due to poor transport through tissue. To predict the effect of treatment schedule on drug efficacy we developed an in silico method that integrates diffusion through tissue and cell binding into a pharmacokinetic model. The model was evaluated with an array of theoretical drugs that had different rates of diffusivity, binding, and clearance. The efficacy of each drug, quantified as the fraction of cells killed, was calculated for twenty dosage schedules. Simulations showed that efficacy strongly depended on tissue transport, with a range of 0.00 to 99.99%, despite each drug having equal plasma areas under the curve (AUC). For most drugs, schedules that increased exposure also increased efficacy. Drugs with fast clearance benefited the most from increasing the number of doses and this was most effective for those with intermediary binding. All drugs with slow diffusivity were ineffective. For a subset of drugs, increasing the number of doses decreased efficacy. This phenomenon was unexpected because, when considering uptake into tissue, sustained plasma levels from multiple doses are generally assumed to be more effective. This counterintuitive decrease in efficacy was caused by drug retention within tumor tissue. These results established a set of rules that suggests how transport parameters affect the efficacy of drugs at different schedules. The two most predominant rules are (1) multiple doses improve efficacy for drugs with fast clearance, fast diffusivity and low to intermediate cell binding; and (2) one dose is most effective for drugs with slow clearance, slow diffusivity or strong cell binding. Understanding the role of tissue transport when determining drug treatment schedules would improve the outcome of preclinical animal experiments and early clinical trials.
Keywords: Cell binding; Chemotherapy; Diffusion; Optimal treatment schedule; Pharmacokinetics; Retention; Tissue transport model.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures
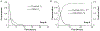




Similar articles
-
Lopinavir/ritonavir: a review of its use in the management of HIV infection.Drugs. 2003;63(8):769-802. doi: 10.2165/00003495-200363080-00004. Drugs. 2003. PMID: 12662125 Review.
-
Rituximab: a review of its use in non-Hodgkin's lymphoma and chronic lymphocytic leukaemia.Drugs. 2003;63(8):803-43. doi: 10.2165/00003495-200363080-00005. Drugs. 2003. PMID: 12662126 Review.
-
Microfluidic technique to measure intratumoral transport and calculate drug efficacy shows that binding is essential for doxorubicin and release hampers Doxil.Integr Biol (Camb). 2013 Sep;5(9):1184-96. doi: 10.1039/c3ib40021b. Integr Biol (Camb). 2013. PMID: 23860772 Free PMC article.
-
Correlation of pharmacokinetics with the antitumor activity of Cetuximab in nude mice bearing the GEO human colon carcinoma xenograft.Cancer Chemother Pharmacol. 2005 Nov;56(5):455-64. doi: 10.1007/s00280-005-1022-3. Epub 2005 Jun 10. Cancer Chemother Pharmacol. 2005. PMID: 15947929
-
Image-based spatio-temporal model of drug delivery in a heterogeneous vasculature of a solid tumor - Computational approach.Microvasc Res. 2019 May;123:111-124. doi: 10.1016/j.mvr.2019.01.005. Epub 2019 Jan 31. Microvasc Res. 2019. PMID: 30711547
Cited by
-
Evaluation of solid tumor response to sequential treatment cycles via a new computational hybrid approach.Sci Rep. 2021 Nov 2;11(1):21475. doi: 10.1038/s41598-021-00989-x. Sci Rep. 2021. PMID: 34728726 Free PMC article.
References
-
- Alley M, Hollingshead M, Dykes D, Waud W, 2004. Human tumor xenograft models in NCI drug development. In: Teicher BA, Andrews PA (Eds.), Anti-cancer Drug Development Guide; Preclinical Screening, Clinical Trials, and Approval. Humana Press, New York, pp. 125–152.
-
- Au JLS, Li D, Gan YB, Gao X, Johnson AL, Johnston J, Millenbaugh NJ, Jang SH, Kuh HJ, Chen CT, Wientjes MG, 1998. Pharmacodynamics of immediate and delayed effects of paclitaxel: Role of slow apoptosis and intracellular drug retention. Cancer Res. 58, 2141–2148. - PubMed
-
- Bryn SR, Dolch GD, 1978. Analysis of binding of daunorubicin and doxorubicin to DNA using computerized curve-fitting procedures. J. Pharmaceutical Sci 67, 688–693. - PubMed
-
- Carlson RW, Sikic BI, 1983. Continuous infusion or bolus injection in cancer chemotherapy. Annals Intern. Med 99, 823–833. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

