Apathy: a neurocircuitry model based on frontotemporal dementia
- PMID: 29066518
- PMCID: PMC6561783
- DOI: 10.1136/jnnp-2017-316277
Apathy: a neurocircuitry model based on frontotemporal dementia
Abstract
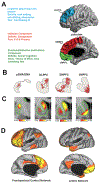
Apathy is a symptom shared among many neurological and psychiatric disorders. However, the underlying neurocircuitry remains incompletely understood. Apathy is one of the core features of behavioural variant frontotemporal dementia (bvFTD), a neurodegenerative disease presenting with heterogeneous combinations of socioaffective symptoms and executive dysfunction. We reviewed all neuroimaging studies of apathy in frontotemporal dementia (FTD) attempting to refine a neurocircuitry model and inform clinical definitions. Levels of apathy have been consistently shown to correlate with the severity of executive dysfunctions across a wide range of diseases, including FTD. Some authors view 'energisation'-the loss of which is central in apathy-as a core executive function. Apathy in FTD is most robustly associated with atrophy, hypometabolism and/or hypoperfusion in the dorsolateral prefrontal cortex, the anterior and middle cingulate cortex, the orbitofrontal cortex and the medial and ventromedial superior frontal gyri. Data also suggest that abnormalities in connecting white matter pathways and functionally connected more posterior cortical areas could contribute to apathy. There is a lack of consistency across studies due to small samples, lenient statistical thresholds, variable measurement scales and the focus on apathy as a unitary concept. Integrating findings across studies, we revise a neurocircuitry model of apathy divided along three subcomponents (cognition/planning, initiation, emotional-affective/motivation) with specific neuroanatomical and cognitive substrates. To increase consistency in clinical practice, a recommendation is made to modify the bvFTD diagnostic criteria of apathy/inertia. More generally, we argue that bvFTD constitutes a disease model to study the neurocircuitry of complex behaviours as a 'lesion-based' approach to neuropsychiatric symptoms observed across diagnostic categories.
Keywords: neuroimaging.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Seeley WW, Zhou J, Kim EJ. Frontotemporal dementia: what can the behavioral variant teach us about human brain organization? Neuroscientist 2012;18:373–85. - PubMed
-
- Harris JM, Gall C, Thompson JC, et al. Sensitivity and specificity of FTDC criteria for behavioral variant frontotemporal dementia. Neurology 2013;80:1881–7. - PubMed
-
- Starkstein SE, Jorge R, Mizrahi R. The prevalence, clinical correlates and treatment of apathy in alzheimer’s disease. The European Journal of Psychiatry 2006;20:96–106.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
