Maternal but Not Infant Anti-HIV-1 Neutralizing Antibody Response Associates with Enhanced Transmission and Infant Morbidity
- PMID: 29066544
- PMCID: PMC5654929
- DOI: 10.1128/mBio.01373-17
Maternal but Not Infant Anti-HIV-1 Neutralizing Antibody Response Associates with Enhanced Transmission and Infant Morbidity
Abstract
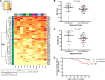
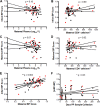
A significant number of infants acquire HIV-1 through their infected mother's breast milk, primarily due to limited access to antiretrovirals. Passive immunization with neutralizing antibodies (nAbs) may prevent this transmission. Previous studies, however, have generated conflicting results about the ability of nAbs to halt mother-to-child transmission (MTCT) and their impact on infant outcomes. This study compared plasma neutralizing activity in exposed infants and the infected mothers (n = 63) against heterologous HIV-1 variants and the quasispecies present in the mother. HIV-exposed uninfected infants (HEU) (n = 42), compared to those that eventually acquired infection (n = 21), did not possess higher nAb responses against heterologous envelopes (P = 0.46) or their mothers' variants (P = 0.45). Transmitting compared to nontransmitting mothers, however, had significantly higher plasma neutralizing activity against heterologous envelopes (P = 0.03), although these two groups did not have significant differences in their ability to neutralize autologous strains (P = 0.39). Furthermore, infants born to mothers with greater neutralizing breadth and potency were significantly more likely to have a serious adverse event (P = 0.03). These results imply that preexisting anti-HIV-1 neutralizing activity does not prevent breast milk transmission. Additionally, high maternal neutralizing breadth and potency may adversely influence both the frequency of breast milk transmission and subsequent infant morbidity.IMPORTANCE Passive immunization trials are under way to understand if preexisting antibodies can decrease mother-to-child HIV-1 transmission and improve infant outcomes. We examined the influence of preexisting maternal and infant neutralizing activity on transmission and infant morbidity in a breastfeeding mother-infant cohort. Neutralization was examined against both the exposure strains circulating in the infected mothers and a standardized reference panel previously used to estimate breadth. HIV-exposed uninfected infants did not possess a broader and more potent response against both the exposure and heterologous strains compared to infants that acquired infection. Transmitting, compared to nontransmitting, mothers had significantly higher neutralization breadth and potency but similar responses against autologous variants. Infants born to mothers with higher neutralization responses were more likely to have a serious adverse event. Our results suggest that preexisting antibodies do not protect against breast milk HIV-1 acquisition and may have negative consequences for the baby.
Keywords: HIV; MTCT; antibodies; breast milk; infant mortality.
Copyright © 2017 Ghulam-Smith et al.
Figures


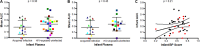

Similar articles
-
Maternal Neutralization-Resistant Virus Variants Do Not Predict Infant HIV Infection Risk.mBio. 2016 Feb 2;7(1):e02221-15. doi: 10.1128/mBio.02221-15. mBio. 2016. PMID: 26838723 Free PMC article.
-
Association of HIV-1 Envelope-Specific Breast Milk IgA Responses with Reduced Risk of Postnatal Mother-to-Child Transmission of HIV-1.J Virol. 2015 Oct;89(19):9952-61. doi: 10.1128/JVI.01560-15. Epub 2015 Jul 22. J Virol. 2015. PMID: 26202232 Free PMC article. Clinical Trial.
-
Maternal Humoral Immune Responses Do Not Predict Postnatal HIV-1 Transmission Risk in Antiretroviral-Treated Mothers from the IMPAACT PROMISE Study.mSphere. 2019 Oct 23;4(5):e00716-19. doi: 10.1128/mSphere.00716-19. mSphere. 2019. PMID: 31645430 Free PMC article. Clinical Trial.
-
Antibodies for prevention of mother-to-child transmission of HIV-1.Curr Opin HIV AIDS. 2015 May;10(3):177-82. doi: 10.1097/COH.0000000000000150. Curr Opin HIV AIDS. 2015. PMID: 25700205 Free PMC article. Review.
-
Transmission of HIV-1 in the face of neutralizing antibodies.Curr HIV Res. 2007 Nov;5(6):578-87. doi: 10.2174/157016207782418461. Curr HIV Res. 2007. PMID: 18045114 Review.
Cited by
-
Broadly neutralizing antibodies: What is needed to move from a rare event in HIV-1 infection to vaccine efficacy?Retrovirology. 2018 Jul 28;15(1):52. doi: 10.1186/s12977-018-0433-2. Retrovirology. 2018. PMID: 30055627 Free PMC article. Review.
-
A Rare Mutation in an Infant-Derived HIV-1 Envelope Glycoprotein Alters Interprotomer Stability and Susceptibility to Broadly Neutralizing Antibodies Targeting the Trimer Apex.J Virol. 2020 Sep 15;94(19):e00814-20. doi: 10.1128/JVI.00814-20. Print 2020 Sep 15. J Virol. 2020. PMID: 32669335 Free PMC article.
-
Pre-existing infant antibody-dependent cellular cytotoxicity associates with reduced HIV-1 acquisition and lower morbidity.Cell Rep Med. 2021 Oct 19;2(10):100412. doi: 10.1016/j.xcrm.2021.100412. eCollection 2021 Oct 19. Cell Rep Med. 2021. PMID: 34755132 Free PMC article.
-
A new cell line for assessing HIV-1 antibody dependent cellular cytotoxicity against a broad range of variants.J Immunol Methods. 2020 May;480:112766. doi: 10.1016/j.jim.2020.112766. Epub 2020 Mar 2. J Immunol Methods. 2020. PMID: 32135162 Free PMC article.
-
HIV Infection and Spread between Th17 Cells.Viruses. 2022 Feb 16;14(2):404. doi: 10.3390/v14020404. Viruses. 2022. PMID: 35215997 Free PMC article. Review.
References
-
- Robert-Guroff M, Oleske JM, Connor EM, Epstein LG, Minnefor AB, Gallo RC. 1987. Relationship between HTLV-III neutralizing antibody and clinical status of pediatric acquired immunodeficiency syndrome (AIDS) and AIDS-related complex cases. Pediatr Res 21:547–550. doi: 10.1203/00006450-198706000-00008. - DOI - PubMed
-
- Ljunggren K, Moschese V, Broliden PA, Giaquinto C, Quinti I, Fenyö EM, Wahren B, Rossi P, Jondal M. 1990. Antibodies mediating cellular cytotoxicity and neutralization correlate with a better clinical stage in children born to human immunodeficiency virus-infected mothers. J Infect Dis 161:198–202. doi: 10.1093/infdis/161.2.198. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

