Role of Granulocyte-Macrophage Colony-Stimulating Factor Production by T Cells during Mycobacterium tuberculosis Infection
- PMID: 29066547
- PMCID: PMC5654932
- DOI: 10.1128/mBio.01514-17
Role of Granulocyte-Macrophage Colony-Stimulating Factor Production by T Cells during Mycobacterium tuberculosis Infection
Abstract
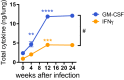
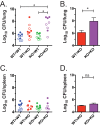
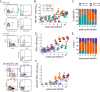
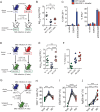
Mice deficient for granulocyte-macrophage colony-stimulating factor (GM-CSF-/-) are highly susceptible to infection with Mycobacterium tuberculosis, and clinical data have shown that anti-GM-CSF neutralizing antibodies can lead to increased susceptibility to tuberculosis in otherwise healthy people. GM-CSF activates human and murine macrophages to inhibit intracellular M. tuberculosis growth. We have previously shown that GM-CSF produced by iNKT cells inhibits growth of M. tuberculosis However, the more general role of T cell-derived GM-CSF during infection has not been defined and how GM-CSF activates macrophages to inhibit bacterial growth is unknown. Here we demonstrate that, in addition to nonconventional T cells, conventional T cells also produce GM-CSF during M. tuberculosis infection. Early during infection, nonconventional iNKT cells and γδ T cells are the main source of GM-CSF, a role subsequently assumed by conventional CD4+ T cells as the infection progresses. M. tuberculosis-specific T cells producing GM-CSF are also detected in the peripheral blood of infected people. Under conditions where nonhematopoietic production of GM-CSF is deficient, T cell production of GM-CSF is protective and required for control of M. tuberculosis infection. However, GM-CSF is not required for T cell-mediated protection in settings where GM-CSF is produced by other cell types. Finally, using an in vitro macrophage infection model, we demonstrate that GM-CSF inhibition of M. tuberculosis growth requires the expression of peroxisome proliferator-activated receptor gamma (PPARγ). Thus, we identified GM-CSF production as a novel T cell effector function. These findings suggest that a strategy augmenting T cell production of GM-CSF could enhance host resistance against M. tuberculosisIMPORTANCEMycobacterium tuberculosis is the bacterium that causes tuberculosis, the leading cause of death by any infection worldwide. T cells are critical components of the immune response to Mycobacterium tuberculosis While gamma interferon (IFN-γ) is a key effector function of T cells during infection, a failed phase IIb clinical trial and other studies have revealed that IFN-γ production alone is not sufficient to control M. tuberculosis In this study, we demonstrate that CD4+, CD8+, and nonconventional T cells produce GM-CSF during Mycobacterium tuberculosis infection in mice and in the peripheral blood of infected humans. Under conditions where other sources of GM-CSF are absent, T cell production of GM-CSF is protective and is required for control of infection. GM-CSF activation of macrophages to limit bacterial growth requires host expression of the transcription factor PPARγ. The identification of GM-CSF production as a T cell effector function may inform future host-directed therapy or vaccine designs.
Keywords: GM-CSF; Mycobacterium tuberculosis; T cells; cytokines; lung infection; macrophages.
Copyright © 2017 Rothchild et al.
Figures


Comment in
-
T Cell Production of GM-CSF Protects the Host during Experimental Tuberculosis.mBio. 2017 Dec 12;8(6):e02087-17. doi: 10.1128/mBio.02087-17. mBio. 2017. PMID: 29233902 Free PMC article.
References
-
- Orme IM. 1987. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J Immunol 138:293–298. - PubMed
-
- Orme IM. 1988. Characteristics and specificity of acquired immunologic memory to Mycobacterium tuberculosis infection. J Immunol 140:3589–3593. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
