Concerted Interneuron Activity in the Cerebellar Molecular Layer During Rhythmic Oromotor Behaviors
- PMID: 29066561
- PMCID: PMC6596751
- DOI: 10.1523/JNEUROSCI.1091-17.2017
Concerted Interneuron Activity in the Cerebellar Molecular Layer During Rhythmic Oromotor Behaviors
Abstract
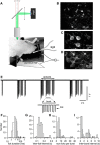
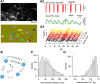
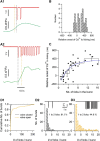
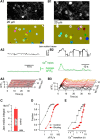
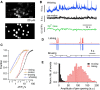
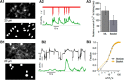
Molecular layer interneurons (MLIs, stellate and basket cells) of the cerebellar cortex are linked together by chemical and electrical synapses and exert a potent feedforward inhibition on Purkinje cells. The functional role of MLIs during specific motor tasks is uncertain. Here, we used two-photon imaging to study the patterns of activity of neighboring individual MLIs in the Crus II region of awake female mice during two types of oromotor activity, licking and bruxing, using specific expression of the genetically encoded calcium indicator protein GCaMP6s. We found that both stellate and basket cells engaged in synchronized waves of calcium activity during licking and bruxing, with high degrees of correlation among the signals collected in individual MLIs. In contrast, no calcium activity was observed during whisking. MLI activity tended to lag behind the onset of sustained licking episodes, indicating a regulatory action of MLIs during licking. Furthermore, during licking, stellate cell activity was anisotropic: the coordination was constant along the direction of parallel fibers (PFs), but fell off with distance along the orthogonal direction. These results suggest a PF drive for Ca2+ signals during licking. In contrast, during bruxing, MLI activity was neither clearly organized spatially nor temporally correlated with oromotor activity. In conclusion, MLI activity exhibits a high degree of correlation both in licking and in bruxing, but spatiotemporal patterns of activity display significant differences for the two types of behavior.SIGNIFICANCE STATEMENT It is known that, during movement, the activity of molecular layer interneurons (MLIs) of the cerebellar cortex is enhanced. However, MLI-MLI interactions are complex because they depend both from excitatory electrical synapses and from potentially inhibitory chemical synapses. Accordingly, the pattern of MLI activity during movement has been unclear. Here, during two oromotor tasks, licking and bruxism, individual neighboring MLIs displayed highly coordinated activity, showing that the positive influences binding MLIs together are predominant. We further find that spatiotemporal patterns differ between licking and bruxing, suggesting that the precise pattern of MLI activity depends on the nature of the motor task.
Keywords: behaving mice; cerebellum; inhibition; interneurons; two-photon imaging.
Copyright © 2017 the authors 0270-6474/17/3711455-14$15.00/0.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
