Activation of STAT3 integrates common profibrotic pathways to promote fibroblast activation and tissue fibrosis
- PMID: 29066712
- PMCID: PMC5654983
- DOI: 10.1038/s41467-017-01236-6
Activation of STAT3 integrates common profibrotic pathways to promote fibroblast activation and tissue fibrosis
Erratum in
-
Author Correction: Activation of STAT3 integrates common profibrotic pathways to promote fibroblast activation and tissue fibrosis.Nat Commun. 2021 Dec 8;12(1):7259. doi: 10.1038/s41467-021-27450-x. Nat Commun. 2021. PMID: 34880239 Free PMC article. No abstract available.
Abstract
Signal transducer and activator of transcription 3 (STAT3) is phosphorylated by various kinases, several of which have been implicated in aberrant fibroblast activation in fibrotic diseases including systemic sclerosis (SSc). Here we show that profibrotic signals converge on STAT3 and that STAT3 may be an important molecular checkpoint for tissue fibrosis. STAT3 signaling is hyperactivated in SSc in a TGFβ-dependent manner. Expression profiling and functional studies in vitro and in vivo demonstrate that STAT3 activation is mediated by the combined action of JAK, SRC, c-ABL, and JNK kinases. STAT3-deficient fibroblasts are less sensitive to the pro-fibrotic effects of TGFβ. Fibroblast-specific knockout of STAT3, or its pharmacological inhibition, ameliorate skin fibrosis in experimental mouse models. STAT3 thus integrates several profibrotic signals and might be a core mediator of fibrosis. Considering that several STAT3 inhibitors are currently tested in clinical trials, STAT3 might be a candidate for molecular targeted therapies of SSc.
Conflict of interest statement
Although none of the authors has any direct conflict of interest related to the use or development of STAT3 inhibitors, O.D. and J.H.W.D. are involved in the development of new targeted therapies for fibrotic diseases such as SSc. O.D. has consultancy relationships and/or has received research funding from Actelion, Pfizer, Ergonex, BMS, Sanofi-Aventis, United BioSource Corporation, Roche/Genentech, Medac, Biovitrium, Boehringer Ingelheim, Novartis, 4D Science, Active Biotech, Bayer, Sinoxa, Serodapharm, EpiPharm, GSK, Pharmacyclics, and Biogen. J.H.W.D. has consultancy relationships with Actelion, Active Biotech, Anamar, Bayer Pharma, Boehringer Ingelheim, Celgene, Galapagos, Inventiva, JB Therapeutics, Medac, Pfizer, RuiYi, and UCB. J.H.W.D. has received research funding from Anamar, Active Biotech, Array Biopharma, BMS, Bayer Pharma, Boehringer Ingelheim, Celgene, GSK, Novartis, Sanofi-Aventis, and UCB. J.H.W.D. is stock owner of 4D Science. The remaining authors declare no competing financial interests.
Figures

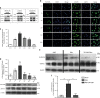

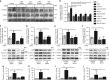
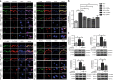
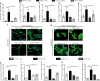



Comment in
-
Systemic sclerosis: STAT3 - A key integrator of profibrotic signalling.Nat Rev Rheumatol. 2017 Nov 21;13(12):693. doi: 10.1038/nrrheum.2017.190. Nat Rev Rheumatol. 2017. PMID: 29158572 No abstract available.
References
-
- Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N. Engl. J. Med. 2009;360:1989–2003. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

