Heparan sulfate: Resilience factor and therapeutic target for cocaine abuse
- PMID: 29066725
- PMCID: PMC5654972
- DOI: 10.1038/s41598-017-13960-6
Heparan sulfate: Resilience factor and therapeutic target for cocaine abuse
Abstract
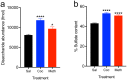
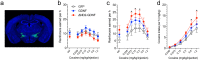
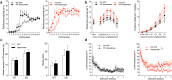
Substance abuse is a pressing problem with few therapeutic options. The identification of addiction resilience factors is a potential strategy to identify new mechanisms that can be targeted therapeutically. Heparan sulfate (HS) is a linear sulfated polysaccharide that is a component of the cell surface and extracellular matrix. Heparan sulfate modulates the activity and distribution of a set of negatively charged signaling peptides and proteins - known as the HS interactome - by acting as a co-receptor or alternative receptor for growth factors and other signaling peptides and sequestering and localizing them, among other actions. Here, we show that stimulants like cocaine and methamphetamine greatly increase HS content and sulfation levels in the lateral hypothalamus and that HS contributes to the regulation of cocaine seeking and taking. The ability of the HS-binding neuropeptide glial-cell-line-derived neurotrophic factor (GDNF) to increase cocaine intake was potentiated by a deletion that abolished its HS binding. The delivery of heparanase, the endo-β-D-glucuronidase that degrades HS, accelerated the acquisition of cocaine self-administration and promoted persistent responding during extinction. Altogether, these results indicate that HS is a resilience factor for cocaine abuse and a novel therapeutic target for the treatment of cocaine addiction.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

