Aberrant glioblastoma neovascularization patterns and their correlation with DCE-MRI-derived parameters following temozolomide and bevacizumab treatment
- PMID: 29066764
- PMCID: PMC5654943
- DOI: 10.1038/s41598-017-14341-9
Aberrant glioblastoma neovascularization patterns and their correlation with DCE-MRI-derived parameters following temozolomide and bevacizumab treatment
Abstract
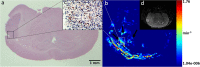
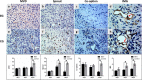
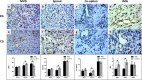
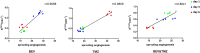
Glioblastoma (GBM) is a highly angiogenic malignancy, and its abundant, aberrant neovascularization is closely related to the proliferation and invasion of tumor cells. However, anti-angiogenesis combined with standard radio-/chemo-therapy produces little improvement in treatment outcomes. Determining the reason for treatment failure is pivotal for GBM treatment. Here, histopathological analysis and dynamic contrast-enhanced MRI (DCE-MRI) were used to explore the effects of temozolomide (TMZ) and bevacizumab (BEV) on GBM neovascularization patterns in an orthotopic U87MG mouse model at 1, 3 and 6 days after treatment. We found that the amount of vascular mimicry (VM) significantly increased 6 days after BEV treatment. TMZ inhibited neovascularization at an early stage, but the microvessel density (MVD) and transfer coefficient (Ktrans) derived from DCE-MRI increased 6 days after treatment. TMZ and BEV combination therapy slightly prolonged the inhibitory effect on tumor microvessels. Sprouting angiogenesis was positively correlated with Ktrans in all treatment groups. The increase in VM after BEV administration and the increase in MVD and Ktrans after TMZ administration may be responsible for treatment resistance. Ktrans holds great potential as an imaging biomarker for indicating the variation in sprouting angiogenesis during drug treatment for GBM.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Nano R, et al. New frontiers for astrocytic tumours. Anticancer Res. 2012;32:2755–2758. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

