Estimating the prevalence of generalized and partial lipodystrophy: findings and challenges
- PMID: 29066925
- PMCID: PMC5604558
- DOI: 10.2147/DMSO.S130810
Estimating the prevalence of generalized and partial lipodystrophy: findings and challenges
Abstract
Background: Lipodystrophy (LD; non-human immunodeficiency virus [HIV]-associated) syndromes are a rare body of disorders for which true prevalence is unknown. Prevalence estimates of rare diseases are important to increase awareness and financial resources. Current qualitative and quantitative estimates of LD prevalence range from ~0.1 to 90 cases/million. We demonstrate an approach to quantitatively estimate LD prevalence (all, generalized, and partial) through a search of 5 electronic medical record (EMR) databases and 4 literature searches.
Methods: EMR and literature searches were conducted from 2012 to 2014. For the EMR database searches (Quintiles, IMS LifeLink, General Electric Healthcare, and Humedica EMR), LD cases were identified by the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code 272.6 (United Kingdom General Practice Research Database used other diagnostic codes to identify LD) plus additional LD-associated clinical characteristics (patients with HIV or documented HIV treatment were excluded). Expert adjudication of cases was used for the Quintiles database only. Literature searches (PubMed and EMBASE) were conducted for each of the 4 major LD subtypes. Prevalence estimates were determined by extrapolating the total number of cases identified for each search to the database population (EMR search) and European population (literature search).
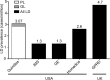
Results: The prevalence range of all LD across all EMR databases was 1.3-4.7 cases/million. For the adjudicated Quintiles search, the estimated prevalence of diagnosed LD was 3.07 cases/million (95% confidence interval [CI], 2.30-4.02), 0.23 cases/million (95% CI, 0.06-0.59) and 2.84 cases/million (95% CI, 2.10-3.75) for generalized lipodystrophy (GL) and partial lipodystrophy (PL), respectively. For all literature searches, the prevalence of all LD in Europe was 2.63 cases/million (0.96 and 1.67 cases/million for GL and PL, respectively).
Conclusion: LD prevalence estimates are at the lower range of previously established numbers, confirming that LD is an ultra-rare disease. The establishment of diagnostic criteria and coding specific to the 4 major LD subtypes and future studies/patient registries are needed to further refine our estimates.
Keywords: adipose tissue; atypical diabetes; dyslipidemia; hypertriglyceridemia; insulin resistance; lipodystrophy; prevalence.
Conflict of interest statement
Disclosure EC is an employee and stockholder of Aegerion Pharmaceuticals. EAO has worked as a consultant/advisor to and has received grant/drug support from Amylin, Bristol-Myers Squibb, AstraZeneca, and Aegerion Pharmaceuticals (manufacturers of metreleptin through the years); Akcea Therapeutics; and Ionis Pharmaceuticals. Unrelated to the field of LD, EAO has received nonmaterial support from Boehringer Ingelheim and grant support from GI Dynamics. EAO is partially supported by R01 DK088114. AG has received research support from Aegerion Pharmaceuticals and Ionis Pharmaceuticals and has worked as a consultant for Aegerion Pharmaceuticals, Biomedical Insights, ClearView Healthcare Partners, Gerson Lehrman Group, and Smith-Solve. DA-V has nothing to disclose. PD is an employee of Complete HEOR Solutions, which has done contract work for Aegerion Pharmaceuticals. The authors report no other conflicts of interest in this work.
Figures


References
-
- EUROPLAN Partners Recommendations for the Development of National Plans for Rare Diseases Guidance Document 20100601. 2010. [Accessed August 23, 2016]. Available from: http://www.europlanproject.eu/Resources/docs/2008-2011_2.EUROPLANRecomme....
-
- Commission of the European Communities Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions on Rare Diseases: Europe’s Challenges. 2008. [Accessed August 23, 2016]. Available from: http://ec.europa.eu/health/ph_threats/non_com/docs/rare_com_en.pdf.
-
- EUR-Lex Regulation (EU) No 536/2014 of the European Parliament and of the Council of 16 April 2014 on Clinical Trials on Medicinal Products for Human Use, and Repealing Directive 2001/20/EC 2014. [Accessed August 23, 2016]. Available from: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32014R0536&....
-
- United States Food and Drug Administration Department of Health and Human Services [webpage on the Internet] 21CFR316.20 – Content and Format of a Request for Orphan-Drug Designation. 2015. [Accessed August 23, 2016]. Available from: http://www.ecfr.gov/cgi-bin/text-idx?SID=ad1729044e3018c44badef3eb3824ef....
-
- European Medicines Agency Points to Consider on the Calculation and Reporting of the Prevalence of a Condition for Orphan Designation. 2002. [Accessed August 24, 2016]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_proc....
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

