An Investigation into Rumen Fungal and Protozoal Diversity in Three Rumen Fractions, during High-Fiber or Grain-Induced Sub-Acute Ruminal Acidosis Conditions, with or without Active Dry Yeast Supplementation
- PMID: 29067009
- PMCID: PMC5641310
- DOI: 10.3389/fmicb.2017.01943
An Investigation into Rumen Fungal and Protozoal Diversity in Three Rumen Fractions, during High-Fiber or Grain-Induced Sub-Acute Ruminal Acidosis Conditions, with or without Active Dry Yeast Supplementation
Abstract
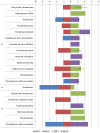
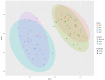
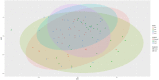
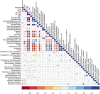
Sub-acute ruminal acidosis (SARA) is a gastrointestinal functional disorder in livestock characterized by low rumen pH, which reduces rumen function, microbial diversity, host performance, and host immune function. Dietary management is used to prevent SARA, often with yeast supplementation as a pH buffer. Almost nothing is known about the effect of SARA or yeast supplementation on ruminal protozoal and fungal diversity, despite their roles in fiber degradation. Dairy cows were switched from a high-fiber to high-grain diet abruptly to induce SARA, with and without active dry yeast (ADY, Saccharomyces cerevisiae) supplementation, and sampled from the rumen fluid, solids, and epimural fractions to determine microbial diversity using the protozoal 18S rRNA and the fungal ITS1 genes via Illumina MiSeq sequencing. Diet-induced SARA dramatically increased the number and abundance of rare fungal taxa, even in fluid fractions where total reads were very low, and reduced protozoal diversity. SARA selected for more lactic-acid utilizing taxa, and fewer fiber-degrading taxa. ADY treatment increased fungal richness (OTUs) but not diversity (Inverse Simpson, Shannon), but increased protozoal richness and diversity in some fractions. ADY treatment itself significantly (P < 0.05) affected the abundance of numerous fungal genera as seen in the high-fiber diet: Lewia, Neocallimastix, and Phoma were increased, while Alternaria, Candida Orpinomyces, and Piromyces spp. were decreased. Likewise, for protozoa, ADY itself increased Isotricha intestinalis but decreased Entodinium furca spp. Multivariate analyses showed diet type was most significant in driving diversity, followed by yeast treatment, for AMOVA, ANOSIM, and weighted UniFrac. Diet, ADY, and location were all significant factors for fungi (PERMANOVA, P = 0.0001, P = 0.0452, P = 0.0068, Monte Carlo correction, respectively, and location was a significant factor (P = 0.001, Monte Carlo correction) for protozoa. Diet-induced SARA shifts diversity of rumen fungi and protozoa and selects against fiber-degrading species. Supplementation with ADY mitigated this reduction in protozoa, presumptively by triggering microbial diversity shifts (as seen even in the high-fiber diet) that resulted in pH stabilization. ADY did not recover the initial community structure that was seen in pre-SARA conditions.
Keywords: SARA; dairy cattle; fungal ITS; mothur; protozoal 18S; rumen pH.
Figures



References
-
- AlZahal O., Li F., Guan L. L., Walker N. D., McBride B. W. (2017). Factors influencing ruminal bacterial community diversity and composition and microbial fibrolytic enzyme abundance in lactating dairy cows with a focus on the role of active dry yeast. J. Dairy Sci. 100, 4377–4393. 10.3168/jds.2016-11473 - DOI - PubMed
-
- Bach A., Iglesias C., Devant M. (2007). Daily rumen pH pattern of loose-housed dairy cattle as affected by feeding pattern and live yeast supplementation. Anim. Feed Sci. Technol. 136, 146–153. 10.1016/j.anifeedsci.2006.09.011 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

