Proof of concept: short-term non-invasive cervical vagus nerve stimulation in patients with drug-refractory gastroparesis
- PMID: 29067158
- PMCID: PMC5641854
- DOI: 10.1136/flgastro-2017-100809
Proof of concept: short-term non-invasive cervical vagus nerve stimulation in patients with drug-refractory gastroparesis
Abstract
Background: Gastric electric stimulation (GES) is a treatment approach to refractory gastroparesis, possibly acting centrally via afferent vagus nerve stimulation (VNS). Non-invasive VNS (nVNS) is a potential alternative to GES that could eliminate the safety risks of or identify likely responders to implantable neurostimulators.
Objective: This open-label proof-of-concept study assessed the effects of nVNS in patients with severe drug-refractory gastroparesis.
Methods: Patients used the Gastroparesis Cardinal Symptom Index (GCSI) to grade symptoms in diaries daily for 2 weeks before treatment (baseline) and during ≥3 weeks of nVNS therapy. Adverse events (AEs) were also diarised. Treatment was self-administered using an nVNS device (gammaCore, electroCore) and consisted of 120 s stimulations to the vagus nerve in the neck (two stimulations to each side three times daily during weeks 1 and 2; three stimulations to each side three times daily during week 3 and beyond). Response was defined as a ≥1 point decrease from baseline in GCSI score.
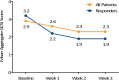
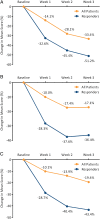
Results: Thirty-five patients enrolled; 23 were compliant with study procedures and were included in the analysis; 7 continued treatment beyond 3 weeks. Response rates were 35% (8/23) at 3 weeks and 43% (10/23) for the duration of therapy (3-6 weeks). For the entire cohort and the 10 responders, improvements from baseline were noted for mean total GCSI and GCSI subscale scores (nausea/vomiting, postprandial fullness/early satiety, bloating). No serious AEs were reported.
Conclusions: These preliminary results provide a signal that nVNS may be useful for treating refractory gastroparesis. Larger controlled studies are warranted.
Keywords: AUTONOMIC NERVOUS SYSTEM; GASTROPARESIS; NERVE - GUT INTERACTIONS.
Conflict of interest statement
Competing interests: DN received a fellowship grant from electroCore. OE participated in a clinical study supported by electroCore. JM has participated in clinical trials supported by electroCore. EL is an employee of electroCore and receives stock ownership.
Figures

References
-
- Parkman HP, Hasler WL, Fisher RS, American Gastroenterological Association. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology 2004;127:1592–622. doi:10.1053/j.gastro.2004.09.055 - DOI - PubMed
-
- Camilleri M, Vazquez-Roque MI. Chapter 4. Gastric dysmotility at the organ level in gastroparesis. In: Parkman HP, McCallum RW. Gastroparesis: pathophysiology, presentation and treatment. New York, NY: Humana Press, 2012:37–46.
-
- Jung HK, Choung RS, Locke GR III, et al. . The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology 2009;136:1225–33. doi:10.1053/j.gastro.2008.12.047 - DOI - PMC - PubMed
-
- Wang YR, Fisher RS, Parkman HP. Gastroparesis-related hospitalizations in the United States: trends, characteristics, and outcomes, 1995–2004. Am J Gastroenterol 2008;103:313–22. doi:10.1111/j.1572-0241.2007.01658.x - DOI - PubMed
-
- Parkman HP, Yates K, Hasler WL, et al. . Clinical features of idiopathic gastroparesis vary with sex, body mass, symptom onset, delay in gastric emptying, and gastroparesis severity. Gastroenterology 2011;140:101–15. doi:10.1053/j.gastro.2010.10.015 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
