Detailed statistical assessment of the characteristics of the ESMO Magnitude of Clinical Benefit Scale (ESMO-MCBS) threshold rules
- PMID: 29067214
- PMCID: PMC5640101
- DOI: 10.1136/esmoopen-2017-000216
Detailed statistical assessment of the characteristics of the ESMO Magnitude of Clinical Benefit Scale (ESMO-MCBS) threshold rules
Abstract
Background: The European Society for Medical Oncology (ESMO) has developed the ESMO Magnitude of Clinical Benefit Scale (ESMO-MCBS), a tool to assess the magnitude of clinical benefit from new cancer therapies. Grading is guided by a dual rule comparing the relative benefit (RB) and the absolute benefit (AB) achieved by the therapy to prespecified threshold values. The ESMO-MCBS v1.0 dual rule evaluates the RB of an experimental treatment based on the lower limit of the 95%CI (LL95%CI) for the hazard ratio (HR) along with an AB threshold. This dual rule addresses two goals: inclusiveness: not unfairly penalising experimental treatments from trials designed with adequate power targeting clinically meaningful relative benefit; and discernment: penalising trials designed to detect a small inconsequential benefit.
Methods: Based on 50 000 simulations of plausible trial scenarios, the sensitivity and specificity of the LL95%CI rule and the ESMO-MCBS dual rule, the robustness of their characteristics for reasonable power and range of targeted and true HRs, are examined. The per cent acceptance of maximal preliminary grade is compared with other dual rules based on point estimate (PE) thresholds for RB.
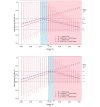
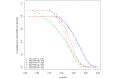
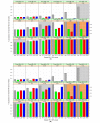
Results: For particularly small or particularly large studies, the observed benefit needs to be relatively big for the ESMO-MCBS dual rule to be satisfied and the maximal grade awarded. Compared with approaches that evaluate RB using the PE thresholds, simulations demonstrate that the MCBS approach better exhibits the desired behaviour achieving the goals of both inclusiveness and discernment.
Conclusions: RB assessment using the LL95%CI for HR rather than a PE threshold has two advantages: it diminishes the probability of excluding big benefit positive studies from achieving due credit and, when combined with the AB assessment, it increases the probability of downgrading a trial with a statistically significant but clinically insignificant observed benefit.
Keywords: Clinical Benefit; ESMO-MCBS; Evaluation of Clinical Benefit; Threshold Rule.
Conflict of interest statement
Competing interests: The authors have declared the following: JB: director of EORTC. EORTC conducts many studies sponsored by, or otherwise supported by, a large number of companies. EORTC is an independent research organisation. MJP: board member: Radius. Consultant (honoraria): AstraZeneca, Lilly, MSD, Novartis, Pfizer, Roche-Genentech, Crescendo Biologics, Periphagen, Huya, Debiopharm and PharmaMar. Research grants to institute: AstraZeneca, Lilly, MDS, Novartis, Pfizer, Roche-Genentech, Synthon, Radius and Servier. Speakers bureau/stock ownership: none. GP: consulting and advisory services, and research support: Amgen, Merck, AstraZeneca, Roche, BMS, MSD and Lilly. J-YD: compensated participation to advisory boards, lecture and symposia: Amgen, Merck Serono, Bayer, Roche/Genentech, Sanofi, AstraZeneca, Boehringer-Ingelheim and Sirtex until April 2016. No further compensated participation in industry events from May 2016 onwards. JT: advisory boards for Amgen, Bayer, Boehringer Ingelheim, Celgene, Chugai, Genentech, Lilly, MSD, Merck Serono, Novartis, Pfizer, Roche, Sanofi, Symphogen, Taiho and Takeda. CCZ: honoraria: AstraZeneca, Celgene, Roche, Novartis, Bristol Myers Squibb, MSD, Ariad and Newgen. EGEdV: currently conducting research sponsored by the following companies: Amgen, Roche/Genentech, Chugai Pharma, Synthon, AstraZeneca, Radius Health, CytomX Therapeutics and Nordic Nanovector (all payments to the institution). Consulting or advisory role for the following companies: Synthon, Medication and Merck (all payments to the institution). Not a member of any speakers' bureau.
Figures


Comment in
-
ESMO-MCBS v1.1: statistical and patient-relevant shortcomings.Ann Oncol. 2018 Apr 1;29(4):1070-1071. doi: 10.1093/annonc/mdy026. Ann Oncol. 2018. PMID: 29360917 No abstract available.
-
Reply to the letter to the editor 'ESMO-MCBS v1.1: statistical and patient relevant shortcomings' by Emprechtinger et al.Ann Oncol. 2018 May 1;29(5):1335-1338. doi: 10.1093/annonc/mdy108. Ann Oncol. 2018. PMID: 29659680 No abstract available.
References
-
- Cherny NI, Sullivan R, Dafni U, et al. . A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies: the European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS). Ann Oncol 2015;26:1547–73. 10.1093/annonc/mdv249 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

