Characterization of the Growth of Chlamydia trachomatis in In Vitro-Generated Stratified Epithelium
- PMID: 29067282
- PMCID: PMC5641298
- DOI: 10.3389/fcimb.2017.00438
Characterization of the Growth of Chlamydia trachomatis in In Vitro-Generated Stratified Epithelium
Abstract
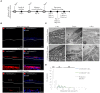
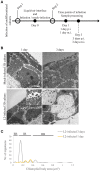
Chlamydia infection targets the mucosal epithelium, where squamous and columnar epithelia can be found. Research on Chlamydia-epithelia interaction has predominantly focused on columnar epithelia, with very little known on how Chlamydia interacts with the squamous epithelium. The stratification and differentiation processes found in the squamous epithelium might influence chlamydial growth and infection dissemination. For this reason, three-dimensional (3D) organotypic stratified squamous epithelial cultures were adapted to mimic the stratified squamous epithelium and chlamydial infection was characterized. Chlamydia trachomatis infection in monolayers and 3D cultures were monitored by immunofluorescence and transmission electron microscopy to evaluate inclusion growth and chlamydial interconversion between elementary and reticulate body. We observed that the stratified epithelium varied in susceptibility to C. trachomatis serovars L2 and D infection. The undifferentiated basal cells were susceptible to infection by both serovars, while the terminally differentiated upper layers were resistant. The differentiating suprabasal cells exhibited different susceptibilities to serovars L2 and D, with the latter unable to establish a successful infection in this layer. Mature elementary body-containing inclusions were much more prevalent in these permissive basal layers, while the uppermost differentiated layers consistently harbored very few reticulate bodies with no elementary bodies, indicative of severely limited bacterial replication and development. For serovar D, the differentiation state of the host cell was a determining factor, as calcium-induced differentiation of cells in a monolayer negatively affected growth of this serovar, in contrast to serovar L2. The apparent completion of the developmental cycle in the basal layers of the 3D cultures correlated with the greater degree of dissemination within and the level of disruption of the stratified epithelium. Our studies indicate that the squamous epithelium is a suboptimal environment for growth, and thus potentially contributing to the protection of the lower genital tract from infection. The relatively more fastidious serovar D exhibited more limited growth than the faster-growing and more invasive L2 strain. However, if given access to the more hospitable basal cell layer, both strains were able to produce mature inclusions, replicate, and complete their developmental cycle.
Keywords: 3D culture; Chlamydia; organotypic cultures; squamous stratified epithelium.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

