Design of a Bayesian adaptive phase 2 proof-of-concept trial for BAN2401, a putative disease-modifying monoclonal antibody for the treatment of Alzheimer's disease
- PMID: 29067290
- PMCID: PMC5644271
- DOI: 10.1016/j.trci.2016.01.001
Design of a Bayesian adaptive phase 2 proof-of-concept trial for BAN2401, a putative disease-modifying monoclonal antibody for the treatment of Alzheimer's disease
Abstract
Introduction: Recent failures in phase 3 clinical trials in Alzheimer's disease (AD) suggest that novel approaches to drug development are urgently needed. Phase 3 risk can be mitigated by ensuring that clinical efficacy is established before initiating confirmatory trials, but traditional phase 2 trials in AD can be lengthy and costly.
Methods: We designed a Bayesian adaptive phase 2, proof-of-concept trial with a clinical endpoint to evaluate BAN2401, a monoclonal antibody targeting amyloid protofibrils. The study design used dose response and longitudinal modeling. Simulations were used to refine study design features to achieve optimal operating characteristics.
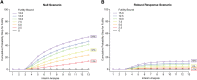
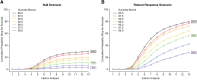
Results: The study design includes five active treatment arms plus placebo, a clinical outcome, 12-month primary endpoint, and a maximum sample size of 800. The average overall probability of success is ≥80% when at least one dose shows a treatment effect that would be considered clinically meaningful. Using frequent interim analyses, the randomization ratios are adapted based on the clinical endpoint, and the trial can be stopped for success or futility before full enrollment.
Discussion: Bayesian statistics can enhance the efficiency of analyzing the study data. The adaptive randomization generates more data on doses that appear to be more efficacious, which can improve dose selection for phase 3. The interim analyses permit stopping as soon as a predefined signal is detected, which can accelerate decision making. Both features can reduce the size and duration of the trial. This study design can mitigate some of the risks associated with advancing to phase 3 in the absence of data demonstrating clinical efficacy. Limitations to the approach are discussed.
Keywords: Adaptive trial; Alzheimer's disease; Bayesian analysis; Clinical trial simulation; Interim analysis; Monoclonal antibody.
Figures

References
-
- Sparks D.L., Sabbagh M.N., Connor D.J., Lopez J., Launer L.J., Browne P. Atorvastatin for the treatment of mild to moderate Alzheimer disease: preliminary results. Arch Neurol. 2005;62:753–757. - PubMed
-
- Feldman H.H., Doody R.S., Kivipelto M., Sparks D.L., Waters D.D., Jones R.W., LEADe Investigators Randomized controlled trial of atorvastatin in mild to moderate Alzheimer disease: LEADe. Neurology. 2010;74:956–964. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
