Suicidal ideation and behavior assessment in dementia studies: An Internet survey
- PMID: 29067294
- PMCID: PMC5644276
- DOI: 10.1016/j.trci.2016.02.002
Suicidal ideation and behavior assessment in dementia studies: An Internet survey
Abstract
Introduction: The AARR task force on suicidal ideation and behavior (SI/SB) in dementia conducted an online survey on the extent of SI/SB in individuals diagnosed with mild cognitive impairment (MCI) or dementia who were participating in clinical trials.
Methods: Investigators with experience in conducting SI/SB assessments in clinical trial subjects with MCI or dementia were invited to complete a global 19-item online survey.
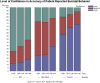
Results: A total of 204 evaluable responses were collected with the majority from North America and Europe (83.4%) and the remainder from Asia, Latin America, and Mideast/Africa. The mean (SD) number of subjects personally assessed by the respondents in the past year with MCI, mild-moderate dementia, or severe dementia was 12.8 (26.2), 31.2 (39.6), and 10.1 (34.7), respectively. The mean number of subjects in each diagnostic group with suicidal ideation (SI), suicidal behavior (SB), or completed suicide (CS) was on average quite low (0.3 to 1.1 for SI, 0.1 to 0.2 for SB, and 0.0 to 0.2 for CS). Confidence in subject self-reports of SI/SB over different time periods declined with increasing severity of cognitive impairment and with increasing duration of the recall time period assessed. Of respondents, 56% and 75% had low confidence in self-ratings of SI/SB from subjects with severe dementia over the past 24 hours and the past week to 1 month, respectively. Ratings of the reliability of information collected on SI/SB also decreased with increasing severity of cognitive impairment. Approximately 70% of respondents rated the reliability of the information they obtained from all sources (patient, caregiver, and others) for subjects with MCI as high, but only about half (42.0% to 55.3%) and less than a quarter (17.4% to 24.3%) rated the reliability of information obtained for subjects with mild to moderate dementia or severe dementia as high, respectively.
Discussion: These results support the usefulness of prospective SI/SB assessments in MCI and mild dementia, raise questions about the reliability of assessments in moderate dementia, and confirm their lack of clinical utility in severe dementia. The results highlight the need for development of validated assessment instruments adapted to the stage of cognitive decline of the patients under study and may be the most effective in the earliest stages of the disease.
Keywords: C-SSRS in dementia trials; Prospective assessment of suicidal ideation and behavior in dementia; Suicidal ideation and behavior in dementia clinical trials.
Figures

References
-
- Guidance for Industry: Suicidality: Prospective Assessment of Occurrence in Clinical Trials. U.S. Department of Health and Human Services. Food and Drug Administration Center for Drug Evaluation and Research (CDER), Division of Psychiatry Products; Silverspring, MD: 2010.
-
- Guidance for Industry: Suicidal Ideation and Behavior: Prospective Assessment of Occurrence in Clinical Trials. U.S. Department of Health and Human Services. Food and Drug Administration Center for Drug Evaluation and Research (CDER), Division of Psychiatry Products; Silverspring, MD: 2012.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
