Profiling the dynamics of CSF and plasma Aβ reduction after treatment with JNJ-54861911, a potent oral BACE inhibitor
- PMID: 29067308
- PMCID: PMC5651349
- DOI: 10.1016/j.trci.2016.08.001
Profiling the dynamics of CSF and plasma Aβ reduction after treatment with JNJ-54861911, a potent oral BACE inhibitor
Abstract
Objectives: Safety, tolerability, pharmacokinetics, and pharmacodynamics of a novel β-site amyloid precursor protein cleaving enzyme 1 (BACE1) inhibitor, JNJ-54861911, were assessed after single and multiple dosing in healthy participants.
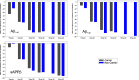
Methods: Two randomized, placebo-controlled, double-blind studies were performed using single and multiple ascending JNJ-54861911 doses (up to 14 days) in young and elderly healthy participants. Regular blood samples and frequent CSF samples, up to 36 hours after last dose, were collected to assess the pharmacokinetic and pharmacodynamic (Aβ, sAPPα,β,total levels) profiles of JNJ-54861911.
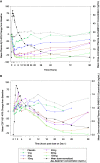
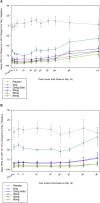
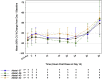
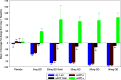
Results: JNJ-54861911 was well-tolerated, adverse events were uncommon and unrelated to JNJ-54861911. JNJ-54861911 showed dose-proportional CSF and plasma pharmacokinetic profiles. Plasma- and CSF-Aβ and CSF-sAPPβ were reduced in a dose-dependent manner. Aβ reductions (up to 95%) outlasted exposure to JNJ-54861911. APOE ε4 carrier status and baseline Aβ levels did not influence Aβ/sAPPβ reductions.
Conclusion: JNJ-54861911, a potent brain-penetrant BACE1 inhibitor, achieved high and stable Aβ reductions after single and multiple dosing in healthy participants.
Keywords: Alzheimer's disease; Amyloid β; BACE inhibitors; BACE1; JNJ-54861911; β-secretase enzyme.
Figures
References
-
- Morris J.C., Price J.L. Pathologic correlates of nondemented aging, mild cognitive impairment, and early-stage Alzheimer's disease. J Mol Neurosci. 2001;17:101–118. - PubMed
-
- Hardy J.A., Higgins G.A. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. - PubMed
-
- Karran E., Mercken M., De Strooper B. The amyloid cascade hypothesis for Alzheimer's disease: an appraisal for the development of therapeutics. Nat Rev Drug Discov. 2011;10:698–712. - PubMed
-
- Sinha S., Anderson J.P., Barbour R., Basi G.S., Caccavello R., Davis D. Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature. 1999;402:537–540. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
