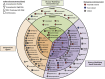
Alzheimer's drug-development pipeline: 2016
- PMID: 29067309
- PMCID: PMC5651348
- DOI: 10.1016/j.trci.2016.07.001
Alzheimer's drug-development pipeline: 2016
Abstract
Background: Alzheimer's disease (AD) is growing in frequency and new therapies are urgently needed.
Methods: We assessed clinicaltrials.gov (accessed 1-4-2016) to determine the number and characteristics of trials in phase I, phase II, and phase III for treatment of AD.
Results: There are currently 24 agents in 36 trials in phase III of AD drug development. Seven of these 24 agents are symptomatic cognitive-enhancing compounds, and 17 are disease-modifying treatments (DMTs). Most DMTs address amyloid-related targets (76%). There are 45 agents in phase II being assessed in 52 clinical trials. Phase II trials include 30 DMTs, with 26 small molecules and 4 immunotherapies. There are 24 agents in the first phase of AD drug development.
Discussion: Amyloid is the principal target of late-stage development programs. There are relatively few agents in clinical trials for AD suggesting a need to amplify the drug discovery ecosystem.
Keywords: Alzheimer's disease; Amyloid; Biomarkers; Cognitive enhancement; Drug development; Phase I; Phase II; Phase III; Tau.
Figures
References
-
- Alzheimer's Association 2015 Alzheimer's disease facts and figures. Alzheimers Dement. 2015;11:332–384. - PubMed
-
- Sosa-Ortiz A.L., Acosta-Castillo I., Prince M.J. Epidemiology of dementias and Alzheimer's disease. Arch Med Res. 2012;43:600–608. - PubMed
-
- Tasneem A., Aberle L., Ananth H., Chakraborty S., Chiswell K., McCourt B.J. The database for aggregate analysis of ClinicalTrials.gov (AACT) and subsequent regrouping by clinical specialty. PLoS One. 2012;7:e33677. - PMC - PubMed
-
- Berk C., Paul G., Sabbagh M. Investigational drugs in Alzheimer's disease: current progress. Expert Opin Investig Drugs. 2014;23:837–846. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources