Zfra restores memory deficits in Alzheimer's disease triple-transgenic mice by blocking aggregation of TRAPPC6AΔ, SH3GLB2, tau, and amyloid β, and inflammatory NF-κB activation
- PMID: 29067327
- PMCID: PMC5651433
- DOI: 10.1016/j.trci.2017.02.001
Zfra restores memory deficits in Alzheimer's disease triple-transgenic mice by blocking aggregation of TRAPPC6AΔ, SH3GLB2, tau, and amyloid β, and inflammatory NF-κB activation
Abstract
Introduction: Zinc finger-like protein that regulates apoptosis (Zfra) is a naturally occurring 31-amino-acid protein. Synthetic peptides Zfra1-31 and Zfra4-10 are known to effectively block the growth of many types of cancer cells.
Methods: Ten-month-old triple-transgenic (3×Tg) mice for Alzheimer's disease (AD) received synthetic Zfra peptides via tail vein injections, followed by examining restoration of memory deficits.
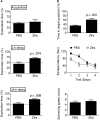
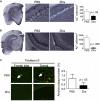
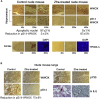
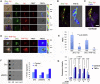
Results: Zfra significantly downregulated TRAPPC6AΔ, SH3GLB2, tau, and amyloid β (Αβ) aggregates in the brains of 3×Tg mice and effectively restored their memory capabilities. Zfra inhibited melanoma-induced neuronal death in the hippocampus and plaque formation in the cortex. Mechanistically, Zfra blocked the aggregation of amyloid β 42 and many serine-containing peptides in vitro, suppressed tumor necrosis factor-mediated NF-κB activation, and bound cytosolic proteins for accelerating their degradation in ubiquitin/proteasome-independent manner.
Discussion: Zfra peptides exhibit a strong efficacy in blocking tau aggregation and amyloid Αβ formation and restore memory deficits in 3×Tg mice, suggesting its potential for treatment of AD.
Keywords: Neurodegeneration; Peptide polymerization; Protein degradation; SH3GLB2; TRAPPC6A; WWOX; Zfra.
Figures



Similar articles
-
Zfra Inhibits the TRAPPC6AΔ-Initiated Pathway of Neurodegeneration.Int J Mol Sci. 2022 Nov 22;23(23):14510. doi: 10.3390/ijms232314510. Int J Mol Sci. 2022. PMID: 36498839 Free PMC article.
-
Zfra Overrides WWOX in Suppressing the Progression of Neurodegeneration.Int J Mol Sci. 2024 Mar 20;25(6):3507. doi: 10.3390/ijms25063507. Int J Mol Sci. 2024. PMID: 38542478 Free PMC article. Review.
-
WWOX Phosphorylation, Signaling, and Role in Neurodegeneration.Front Neurosci. 2018 Aug 15;12:563. doi: 10.3389/fnins.2018.00563. eCollection 2018. Front Neurosci. 2018. PMID: 30158849 Free PMC article. Review.
-
WWOX and Its Binding Proteins in Neurodegeneration.Cells. 2021 Jul 14;10(7):1781. doi: 10.3390/cells10071781. Cells. 2021. PMID: 34359949 Free PMC article. Review.
-
Zfra activates memory Hyal-2+ CD3- CD19- spleen cells to block cancer growth, stemness, and metastasis in vivo.Oncotarget. 2015 Feb 28;6(6):3737-51. doi: 10.18632/oncotarget.2895. Oncotarget. 2015. PMID: 25686832 Free PMC article.
Cited by
-
WWOX inhibition by Zfra1-31 restores mitochondrial homeostasis and viability of neuronal cells exposed to high glucose.Cell Mol Life Sci. 2022 Aug 19;79(9):487. doi: 10.1007/s00018-022-04508-7. Cell Mol Life Sci. 2022. PMID: 35984507 Free PMC article.
-
WWOX Possesses N-Terminal Cell Surface-Exposed Epitopes WWOX7-21 and WWOX7-11 for Signaling Cancer Growth Suppression and Prevention In Vivo.Cancers (Basel). 2019 Nov 19;11(11):1818. doi: 10.3390/cancers11111818. Cancers (Basel). 2019. PMID: 31752354 Free PMC article.
-
Novel Homozygous Mutation in the WWOX Gene Causes Seizures and Global Developmental Delay: Report and Review.Transl Neurosci. 2018 Dec 31;9:203-208. doi: 10.1515/tnsci-2018-0029. eCollection 2018. Transl Neurosci. 2018. PMID: 30746283 Free PMC article.
-
A p53/TIAF1/WWOX triad exerts cancer suppression but may cause brain protein aggregation due to p53/WWOX functional antagonism.Cell Commun Signal. 2019 Jul 17;17(1):76. doi: 10.1186/s12964-019-0382-y. Cell Commun Signal. 2019. PMID: 31315632 Free PMC article.
-
Causal differential expression analysis under unmeasured confounders with causarray.bioRxiv [Preprint]. 2025 Feb 5:2025.01.30.635593. doi: 10.1101/2025.01.30.635593. bioRxiv. 2025. PMID: 39975097 Free PMC article. Preprint.
References
-
- Hsu L.J., Schultz L., Mattison J., Lin Y.S., Chang N.S. Cloning and characterization of a small-size peptide Zfra that regulates the cytotoxic function of tumor necrosis factor by interacting with JNK1. Biochem Biophys Res Commun. 2005;327:415–423. - PubMed
-
- Hsu L.J., Hong Q., Schultz L., Kuo E., Lin S.R., Lee M.H. Zfra is an inhibitor of Bcl-2 expression and cytochrome c release from the mitochondria. Cell Signal. 2008;20:1303–1312. - PubMed
-
- Chang N.S., Hsu L.J., Lin Y.S., Lai F.J., Sheu H.M. WW domain-containing oxidoreductase: a candidate tumor suppressor. Trends Mol Med. 2007;13:12–22. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
