UB-311, a novel UBITh® amyloid β peptide vaccine for mild Alzheimer's disease
- PMID: 29067332
- PMCID: PMC5651432
- DOI: 10.1016/j.trci.2017.03.005
UB-311, a novel UBITh® amyloid β peptide vaccine for mild Alzheimer's disease
Abstract
Introduction: A novel amyloid β (Aβ) synthetic peptide vaccine (UB-311) has been evaluated in a first-in-human trial with patients of mild-to-moderate Alzheimer's disease. We describe translational research covering vaccine design, preclinical characterization, and phase-I clinical trial with supportive outcome that advances UB-311 into an ongoing phase-II trial.
Methods: UB-311 is constructed with two synthetic Aβ1-14-targeting peptides (B-cell epitope), each linked to different helper T-cell peptide epitopes (UBITh®) and formulated in a Th2-biased delivery system. The hAPP751 transgenic mouse model was used to perform the proof-of-concept study. Baboons and macaques were used for preclinical safety, tolerability, and immunogenicity evaluation. Patients with mild-to-moderate Alzheimer's disease (AD) were immunized by intramuscular route with 3 doses of UB-311 at weeks 0, 4, and 12, and monitored until week 48. Safety and immunogenicity were assessed per protocol, and preliminary efficacy was analyzed by Alzheimer's Disease Assessment Scale-Cognitive Subscale (ADAS-Cog), Mini-Mental State Examination (MMSE), and Alzheimer's Disease Cooperative Study-Clinician's Global Impression of Change (ADCS-CGIC).
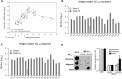
Results: UB-311 covers a diverse genetic background and facilitates strong immune response with high responder rate. UB-311 reduced the levels of Aβ1-42 oligomers, protofibrils, and plaque load in hAPP751 transgenic mice. Safe and well-tolerated UB-311 generated considerable site-specific (Aβ1-10) antibodies across all animal species examined. In AD patients, UB-311 induced a 100% responder rate; injection site swelling and agitation were the most common adverse events (4/19 each). A slower rate of increase in ADAS-Cog from baseline to week 48 was observed in the subgroup of mild AD patients (MMSE ≥ 20) compared with the moderate AD subgroup, suggesting that UB-311 may have a potential of cognition improvement in patients with early stage of Alzheimer's dementia.
Discussion: The UBITh® platform can generate a high-precision molecular vaccine with high responder rate, strong on-target immunogenicity, and a potential of cognition improvement, which support UB-311 for active immunotherapy in early-to-mild AD patients currently enrolled in a phase-II trial (NCT02551809).
Keywords: Alzheimer's disease; Amyloid β vaccine; FIH clinical trial; UB-311; UBITh® platform.
Figures


References
-
- Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. - PubMed
-
- Kayed R., Lasagna-Reeves C.A. Molecular mechanisms of amyloid oligomers toxicity. J Alzheimers Dis. 2013;33:S67–S78. - PubMed
-
- Qian X.X., Hamad B., Dias-Lalcaca G. The Alzheimer disease market. Nat Rev Drug Discov. 2015;14:675–676. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
