A randomized feasibility pilot trial of hearing treatment for reducing cognitive decline: Results from the Aging and Cognitive Health Evaluation in Elders Pilot Study
- PMID: 29067347
- PMCID: PMC5651440
- DOI: 10.1016/j.trci.2017.06.003
A randomized feasibility pilot trial of hearing treatment for reducing cognitive decline: Results from the Aging and Cognitive Health Evaluation in Elders Pilot Study
Abstract
Introduction: Hearing loss (HL) is prevalent and independently related to cognitive decline and dementia. There has never been a randomized trial to test if HL treatment could reduce cognitive decline in older adults.
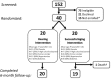
Methods: A 40-person (aged 70-84 years) pilot study in Washington County, MD, was conducted. Participants were randomized 1:1 to a best practices hearing or successful aging intervention and followed for 6 months. clinicaltrials.gov Identifier: NCT02412254.
Results: The Aging and Cognitive Health Evaluation in Elders Pilot (ACHIEVE-P) Study demonstrated feasibility in recruitment, retention, and implementation of interventions with no treatment-related adverse events. A clear efficacy signal of the hearing intervention was observed in perceived hearing handicap (mean of 0.11 to -1.29 standard deviation [SD] units; lower scores better) and memory (mean of -0.10 SD to 0.38 SD).
Discussion: ACHIEVE-P sets the stage for the full-scale ACHIEVE trial (N = 850, recruitment beginning November 2017), the first randomized trial to determine efficacy of a best practices hearing (vs. successful aging) intervention on reducing cognitive decline in older adults with HL.
Keywords: Clinical trials; Cognition; Dementia; Epidemiology; Hearing; Longitudinal study; Memory; Presbycusis.
Figures
References
Associated data
Grants and funding
- HHSN268201100012C/HL/NHLBI NIH HHS/United States
- HHSN268201100009I/HL/NHLBI NIH HHS/United States
- U01 HL096812/HL/NHLBI NIH HHS/United States
- HHSN268201100010C/HL/NHLBI NIH HHS/United States
- HHSN268201100008C/HL/NHLBI NIH HHS/United States
- HHSN268201100007C/HL/NHLBI NIH HHS/United States
- HHSN268201100011I/HL/NHLBI NIH HHS/United States
- HHSN268201100011C/HL/NHLBI NIH HHS/United States
- HHSN268201100006C/HL/NHLBI NIH HHS/United States
- HHSN268201100005I/HL/NHLBI NIH HHS/United States
- U01 HL096814/HL/NHLBI NIH HHS/United States
- P50 AG005146/AG/NIA NIH HHS/United States
- HHSN268201100005G/HL/NHLBI NIH HHS/United States
- U01 HL096917/HL/NHLBI NIH HHS/United States
- HHSN268201100008I/HL/NHLBI NIH HHS/United States
- U01 HL096902/HL/NHLBI NIH HHS/United States
- R34 AG046548/AG/NIA NIH HHS/United States
- HHSN268201100009C/HL/NHLBI NIH HHS/United States
- R01 HL070825/HL/NHLBI NIH HHS/United States
- HHSN268201100005C/HL/NHLBI NIH HHS/United States
- U01 HL096899/HL/NHLBI NIH HHS/United States
- HHSN268201100007I/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical