Effect of Therapeutic Hypothermia Initiated After 6 Hours of Age on Death or Disability Among Newborns With Hypoxic-Ischemic Encephalopathy: A Randomized Clinical Trial
- PMID: 29067428
- PMCID: PMC5783566
- DOI: 10.1001/jama.2017.14972
Effect of Therapeutic Hypothermia Initiated After 6 Hours of Age on Death or Disability Among Newborns With Hypoxic-Ischemic Encephalopathy: A Randomized Clinical Trial
Erratum in
-
Author Added to Byline.JAMA. 2018 Mar 13;319(10):1051. doi: 10.1001/jama.2018.1657. JAMA. 2018. PMID: 29536079 Free PMC article. No abstract available.
Abstract
Importance: Hypothermia initiated at less than 6 hours after birth reduces death or disability for infants with hypoxic-ischemic encephalopathy at 36 weeks' or later gestation. To our knowledge, hypothermia trials have not been performed in infants presenting after 6 hours.
Objective: To estimate the probability that hypothermia initiated at 6 to 24 hours after birth reduces the risk of death or disability at 18 months among infants with hypoxic-ischemic encephalopathy.
Design, setting, and participants: A randomized clinical trial was conducted between April 2008 and June 2016 among infants at 36 weeks' or later gestation with moderate or severe hypoxic-ischemic encephalopathy enrolled at 6 to 24 hours after birth. Twenty-one US Neonatal Research Network centers participated. Bayesian analyses were prespecified given the anticipated limited sample size.
Interventions: Targeted esophageal temperature was used in 168 infants. Eighty-three hypothermic infants were maintained at 33.5°C (acceptable range, 33°C-34°C) for 96 hours and then rewarmed. Eighty-five noncooled infants were maintained at 37.0°C (acceptable range, 36.5°C-37.3°C).
Main outcomes and measures: The composite of death or disability (moderate or severe) at 18 to 22 months adjusted for level of encephalopathy and age at randomization.
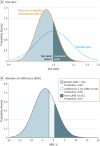
Results: Hypothermic and noncooled infants were term (mean [SD], 39 [2] and 39 [1] weeks' gestation, respectively), and 47 of 83 (57%) and 55 of 85 (65%) were male, respectively. Both groups were acidemic at birth, predominantly transferred to the treating center with moderate encephalopathy, and were randomized at a mean (SD) of 16 (5) and 15 (5) hours for hypothermic and noncooled groups, respectively. The primary outcome occurred in 19 of 78 hypothermic infants (24.4%) and 22 of 79 noncooled infants (27.9%) (absolute difference, 3.5%; 95% CI, -1% to 17%). Bayesian analysis using a neutral prior indicated a 76% posterior probability of reduced death or disability with hypothermia relative to the noncooled group (adjusted posterior risk ratio, 0.86; 95% credible interval, 0.58-1.29). The probability that death or disability in cooled infants was at least 1%, 2%, or 3% less than noncooled infants was 71%, 64%, and 56%, respectively.
Conclusions and relevance: Among term infants with hypoxic-ischemic encephalopathy, hypothermia initiated at 6 to 24 hours after birth compared with noncooling resulted in a 76% probability of any reduction in death or disability, and a 64% probability of at least 2% less death or disability at 18 to 22 months. Hypothermia initiated at 6 to 24 hours after birth may have benefit but there is uncertainty in its effectiveness.
Trial registration: clinicaltrials.gov Identifier: NCT00614744.
Conflict of interest statement
Figures
Comment in
-
Bayesian Analysis: Using Prior Information to Interpret the Results of Clinical Trials.JAMA. 2017 Oct 24;318(16):1605-1606. doi: 10.1001/jama.2017.15574. JAMA. 2017. PMID: 29067406 No abstract available.
-
Does late therapeutic hypothermia reduce risk of death or disability?Acta Paediatr. 2018 Jun;107(6):1103. doi: 10.1111/apa.14334. Epub 2018 Apr 19. Acta Paediatr. 2018. PMID: 29672925 Free PMC article. No abstract available.
References
-
- Neonatal Encephalopathy and Neurologic Outcome. 2nd. Washington, DC: American College of Obstetricians and Gynecologists; 2014. American College of Obstetricians and Gynecologists, American Academy of Pediatrics.
-
- Volpe JJ. Neonatal encephalopathy: an inadequate term for hypoxic-ischemic encephalopathy. Ann Neurol. 2012;72(2):156–166. - PubMed
-
- Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365(9460):663–670. - PubMed
-
- Shankaran S, Laptook AR, Ehrenkranz RA, et al. National Institute of Child Health and Human Development Neonatal Research Network Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353(15):1574–1584. - PubMed
-
- Azzopardi DV, Strohm B, Edwards AD, et al. TOBY Study Group Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361(14):1349–1358. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

