Engineered D2R Variants Reveal the Balanced and Biased Contributions of G-Protein and β-Arrestin to Dopamine-Dependent Functions
- PMID: 29068002
- PMCID: PMC5854808
- DOI: 10.1038/npp.2017.254
Engineered D2R Variants Reveal the Balanced and Biased Contributions of G-Protein and β-Arrestin to Dopamine-Dependent Functions
Abstract
The dopamine D2 receptor (D2R), like many G-protein-coupled receptors, signals through G-protein- and β-arrestin-dependent pathways. Preferential activation of one of these pathways is termed functional selectivity or biased signaling and is a promising therapeutic strategy. Though biased signaling through D2Rs has been demonstrated, acquiring the mechanistic details of biased D2R/G-protein and D2R/β-arrestin signaling in vivo has been challenging because of the lack of techniques that specifically target these interactions in discrete cell populations. To address this question, we employed a cell type-specific viral expression approach to restore D2R variants that preferentially engage either G-protein or β-arrestin signaling in 'indirect pathway' medium spiny neurons (iMSNs), because of their central role in dopamine circuitry. We found that the effect of haloperidol antagonism on D2R metabolic signaling events is largely mediated by acute blockade of D2R/G-protein signaling. We show that a D2R-driven behavior, nestlet shredding, is similarly driven by D2R/G-protein signaling. On the other hand, D2R-driven locomotion and rearing require coordinated D2R/G-protein and D2R/β-arrestin signaling. The acute locomotor response to amphetamine and cocaine similarly depend on both G-protein and β-arrestin D2R signaling. Surprisingly, another psychotropic drug, phencyclidine, displayed a selective D2R/β-arrestin potentiation of locomotion. These findings highlight how D2R mostly relies upon balanced G-protein and β-arrestin signaling in iMSNs. However, the response to haloperidol and phencyclidine indicates that normal D2R signaling homeostasis can be dramatically altered, indicating that targeting a specific D2R signal transduction pathway could allow for more precise modulation of dopamine circuit function.
Figures

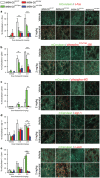

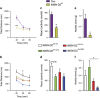
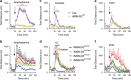
References
-
- Alexander GE, DeLong MR, Strick PL (1986). Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9: 357–381. - PubMed
-
- Bahrami S, Drablos F (2016). Gene regulation in the immediate-early response process. Adv Biol Regul 62: 37–49. - PubMed
-
- Baik JH, Picetti R, Saiardi A, Thiriet G, Dierich A, Depaulis A et al (1995). Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature 377: 424–428. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

