Structure, function, and regulation of mitofusin-2 in health and disease
- PMID: 29068134
- PMCID: PMC6446723
- DOI: 10.1111/brv.12378
Structure, function, and regulation of mitofusin-2 in health and disease
Abstract
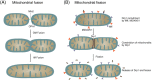
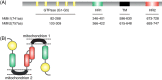
Mitochondria are highly dynamic organelles that constantly migrate, fuse, and divide to regulate their shape, size, number, and bioenergetic function. Mitofusins (Mfn1/2), optic atrophy 1 (OPA1), and dynamin-related protein 1 (Drp1), are key regulators of mitochondrial fusion and fission. Mutations in these molecules are associated with severe neurodegenerative and non-neurological diseases pointing to the importance of functional mitochondrial dynamics in normal cell physiology. In recent years, significant progress has been made in our understanding of mitochondrial dynamics, which has raised interest in defining the physiological roles of key regulators of fusion and fission and led to the identification of additional functions of Mfn2 in mitochondrial metabolism, cell signalling, and apoptosis. In this review, we summarize the current knowledge of the structural and functional properties of Mfn2 as well as its regulation in different tissues, and also discuss the consequences of aberrant Mfn2 expression.
Keywords: Charcot-Marie-Tooth disease; diabetes; mitochondria; mitochondrial dynamics; mitofusin-1; mitofusin-2; neurodegenerative disease; obesity; vascular disease.
© 2017 The Authors. Biological Reviews published by John Wiley & Sons Ltd on behalf of Cambridge Philosophical Society.
Figures

References
-
- Alexander, C. , Votruba, M. , Pesch, U. E. , Thiselton, D. L. , Mayer, S. , Moore, A. , Rodriguez, M. , Kellner, U. , Leo‐Kottler, B. , Auburger, G. , Bhattacharya, S. S. & Wissinger, B. (2000). OPA1, encoding a dynamin‐related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nature Genetics 26, 211–215. - PubMed
-
- Amiott, E. A. , Lott, P. , Soto, J. , Kang, P. B. , McCaffery, J. M. , DiMauro, S. , Abel, E. D. , Flanigan, K. M. , Lawson, V. H. & Shaw, J. M. (2008). Mitochondrial fusion and function in Charcot‐Marie‐Tooth type 2A patient fibroblasts with mitofusin 2 mutations. Experimental Neurology 211, 115–127. - PMC - PubMed
-
- Anton, F. , Dittmar, G. , Langer, T. & Escobar‐Henriques, M. (2013). Two deubiquitylases act on mitofusin and regulate mitochondrial fusion along independent pathways. Molecular Cell 49, 487–498. - PubMed
-
- Arany, Z. , He, H. , Lin, J. , Hoyer, K. , Handschin, C. , Toka, O. , Ahmad, F. , Matsui, T. , Chin, S. , Wu, P. H. , Rybkin, I. I. , Shelton, J. M. , Manieri, M. , Cinti, S. , Schoen, F. J. , et al. (2005). Transcriptional coactivator PGC‐1α controls the energy state and contractile function of cardiac muscle. Cell Metabolism 1, 259–271. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

