ortho-Quinones and Analogues Thereof: Highly Reactive Intermediates for Fast and Selective Biofunctionalization
- PMID: 29068513
- PMCID: PMC5900998
- DOI: 10.1002/chem.201703919
ortho-Quinones and Analogues Thereof: Highly Reactive Intermediates for Fast and Selective Biofunctionalization
Abstract
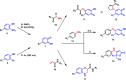
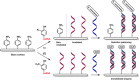
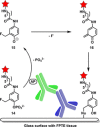
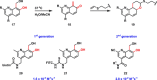
Fast, selective and facile functionalization of biologically relevant molecules is a pursuit of ever-growing importance. A promising approach in this regard employs the high reactivity of quinone and quinone analogues for fast conjugation chemistry by nucleophilic addition or cycloadditions. Combined with in situ generation of these compounds, selective conjugation on proteins and surfaces can be uniquely induced in a time and spatially resolved manner: generation of a quinone can often be achieved by simple addition of an enzyme or stoichiometric amounts of chemoselective oxidant, or by exposure to light. In this Minireview, we discuss the generation and subsequent functionalization of quinones, iminoquinones, and quinone methides. We also discuss practical applications regarding these conjugation strategies.
Keywords: addition reactions; biochemistry; heterocycles; nucleophiles; quinones.
© 2017 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Figures
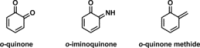
References
-
- Johnsson N., Johnsson K., Acs Chem. Biol. 2007, 2, 31–38. - PubMed
-
- None
-
- Zhu H., Snyder M., Curr. Opin. Chem. Biol. 2003, 7, 55–63; - PubMed
-
- Chari R. V., Miller M. L., Widdison W. C., Angew. Chem. Int. Ed. 2014, 53, 3796–3827; - PubMed
- Angew. Chem. 2014, 126, 3872–3904.
-
- Rusmini F., Zhong Z. Y., Feijen J., Biomacromolecules 2007, 8, 1775–1789. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

