Direct, Mild, and General n-Bu4NBr-Catalyzed Aldehyde Allylsilylation with Allyl Chlorides
- PMID: 29068688
- PMCID: PMC5693333
- DOI: 10.1021/acs.orglett.7b03193
Direct, Mild, and General n-Bu4NBr-Catalyzed Aldehyde Allylsilylation with Allyl Chlorides
Abstract
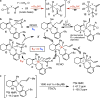
A direct, mild, and general method for the enantioselective allylsilylation of aldehydes with allyl chlorides is reported. The reactions are effectively catalyzed by 5 mol % of n-Bu4NBr, and this rate acceleration allows the use of complex allyl donors in fragment-coupling reactions and of electron-deficient allyl donors. The results are (1) significant progress toward a "universal" asymmetric aldehyde allylation reaction that can reliably and highly stereoselectively couple any allyl chloride_aldehyde combination and (2) the discovery of a novel mode of nucleophilic catalysis for aldehyde allylsilylation reactions.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Denmark SE, Almstead NG. In: Modern Carbonyl Chemistry. Otera J, editor. Wiley-VCH; Weinheim, Germany: 2000. Chapter 10.
- Chemler SR, Roush WR. In: Modern Carbonyl Chemistry. Otera J, editor. Wiley-VCH; Weinheim, Germany: 2000. Chapter 11.
-
- Yamamoto Y, Asao N. Chem Rev. 1993;93:2207–2293.
- Masse CE, Panek JS. Chem Rev. 1995;95:1293–1316.
- Ramachandran PV. Aldrichimica Acta. 2002;35:23–35.
- Denmark SE, Fu J. Chem Rev. 2003;103:2763–2794. - PubMed
- Kennedy JWJ, Hall DG. Angew Chem Int Ed. 2003;42:4732–4739. - PubMed
- Hall DG. Synlett. 2007:1644–1655.
- Lachance H, Hall DG. Org React. 2008;73:1–573.
- Leighton JL. Aldrichimica Acta. 2010;43:3–12.
- Yus M, González-Gómez JC, Foubelo F. Chem Rev. 2011;111:7774–7854. - PubMed
-
- Brown HC, Bhat KS. J Am Chem Soc. 1986;108:293–294. - PubMed
- Brown HC, Bhat KS. J Am Chem Soc. 1986;108:5919–5923. - PubMed
- Garcia J, Kim BM, Masamune S. J Org Chem. 1987;52:4831–4832.
- Roush WR, Ando K, Powers DB, Palkowitz AD, Halterman RL. J Am Chem Soc. 1990;112:6339–6348.
- Roush WR, Palkowitz AD, Ando K. J Am Chem Soc. 1990;112:6348–6359.
- Hafner A, Duthlaer RO, Marti R, Rihs G, Rothe-Streit P, Schwarzenbach F. J Am Chem Soc. 1992;114:2321–2336.
- Jain NF, Takenaka N, Panek JS. J Am Chem Soc. 1996;118:12475–12476.
- Denmark SE, Fu J. J Am Chem Soc. 2001;123:9488–9489. - PubMed
- Lachance H, Lu X, Gravel M, Hall DG. J Am Chem Soc. 2003;125:10160–10161. - PubMed
- Burgos CH, Canales E, Matos K, Soderquist JA. J Am Chem Soc. 2005;127:8044–8049. - PubMed
- Kim H, Ho S, Leighton JL. J Am Chem Soc. 2011;133:6517–6520. - PMC - PubMed
-
- Kim IS, Ngai MY, Krische MJ. J Am Chem Soc. 2008;130:6340–6341. - PMC - PubMed
- Kim IS, Ngai MY, Krische MJ. J Am Chem Soc. 2008;130:14891–14899. - PMC - PubMed
- Kim IS, Han SB, Krische MJ. J Am Chem Soc. 2009;131:2514–2520. - PMC - PubMed
- Han SB, Han H, Krische MJ. J Am Chem Soc. 2010;132:1760–1761. - PMC - PubMed
- Zhang YJ, Yang JH, Kim SH, Krische MJ. J Am Chem Soc. 2010;132:4562–4563. - PMC - PubMed
- Han SB, Gao X, Krische MJ. J Am Chem Soc. 2010;132:9153–9156. - PMC - PubMed
- Gao X, Zhang YJ, Krische MJ. Angew Chem Int Ed. 2011;50:4173–4175. - PMC - PubMed
- Hassan A, Townsend IA, Krische MJ. Chem Commun. 2011;47:10028–10030. - PMC - PubMed
- Gao X, Han H, Krische MJ. J Am Chem Soc. 2011;133:12795–12800. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

