Spatiotemporal changes in the distribution of LHFPL5 in mice cochlear hair bundles during development and in the absence of PCDH15
- PMID: 29069081
- PMCID: PMC5656302
- DOI: 10.1371/journal.pone.0185285
Spatiotemporal changes in the distribution of LHFPL5 in mice cochlear hair bundles during development and in the absence of PCDH15
Abstract
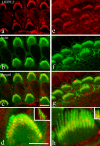
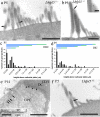
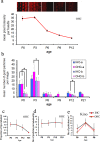
Mechanosensory transduction by vertebrate hair cells depends on a protein complex at the tips of shorter stereocilia associated with mechanoelectrical transduction channels activated by tip links in the hair bundle. In mammalian hair cells, this complex includes transmembrane channel-like protein subunit 1 (TMC1), lipoma HMGIC fusion partner-like 5 protein (LHFPL5) and protocadherin 15 (PCDH15), a lower-end component of the tip link. TMC1 interacts with LHFPL5 and PCDH15 but how the complex develops to maturity, and the relationships between these proteins, remains uncertain. Here we evaluate the spatiotemporal development of LHFPL5 distributions in mouse cochlear hair bundles by immunofluorescence and immunogold transmission electron microscopy, from postnatal day 0 (P0) through P21 in wild type and PCDH15-deficient mice. At P0, hair bundles contain many short microvilli-like processes which we term unranked stereocilia, and a subset of lengthening rows, adjacent to a kinocilium. LHFPL5 is distributed throughout the bundle, including on stereocilia tips and the kinocilium. At P3, 4-to-6 rows of ranked stereocilia are evident, total LHFPL5 expression peaks, and LHFPL5 is localised to ranked stereocilia tips of all rows and to lower shaft/ankle links. By P12, the bundle has a mature pattern with 3 ranked rows but virtually no unranked stereocilia or kinocilium; LHFPL5 expression has declined and become restricted to the tips of shorter stereocilia. Throughout development from P0, expression of LHFPL5 is greater overall on apical than basal bundles, but there is, on average, an equal amount of labelling per labelled tip. In P3 mice lacking PCDH15, LHFPL5 labelling is not at the tips but is primarily on unranked stereocilia and lower lateral links. These data show that LHFPL5 is already present in the MET apparatus at P0 but requires PCDH15 at P3 to remain there. Shaft/ankle link localisation suggests it interacts with link proteins other than PCDH15.
Conflict of interest statement
Figures




References
-
- Hackney CM, Furness DN. The composition and role of cross links in mechanoelectrical transduction in vertebrate sensory hair cells. J Cell Sci. 2013; 126(Pt 8):1721–1731 doi: 10.1242/jcs.106120 - DOI - PubMed
-
- Pickles JO, Comis SD, Osborne MP. Cross-links between stereocilia in the guinea pig organ of Corti, and their possible relation to sensory transduction. Hear Res. 1984; 15(2):103–112. - PubMed
-
- Furness DN, Hackney CM. Cross-links between stereocilia in the guinea pig cochlea. Hear Res. 1985; 18(2):177–188. - PubMed
-
- Beurg M, Fettiplace R, Nam JH, Ricci AJ. Localization of inner hair cell mechanotransducer channels using high-speed calcium imaging. Nat Neurosci. 2009; 12(5):553–558. doi: 10.1038/nn.2295 - DOI - PMC - PubMed
-
- Söllner C1, Rauch GJ, Siemens J, Geisler R, Schuster SC, Müller U et al. Mutations in cadherin 23 affect tip links in zebrafish sensory hair cells. Nature. 2004; 428(6986): 955–959. doi: 10.1038/nature02484 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

