The Edinburgh Consensus: preparing for the advent of disease-modifying therapies for Alzheimer's disease
- PMID: 29070066
- PMCID: PMC5657110
- DOI: 10.1186/s13195-017-0312-4
The Edinburgh Consensus: preparing for the advent of disease-modifying therapies for Alzheimer's disease
Erratum in
-
Correction to: The Edinburgh Consensus: preparing for the advent of disease-modifying therapies for Alzheimer's disease.Alzheimers Res Ther. 2018 Jul 30;10(1):73. doi: 10.1186/s13195-018-0372-0. Alzheimers Res Ther. 2018. PMID: 30060761 Free PMC article.
Abstract
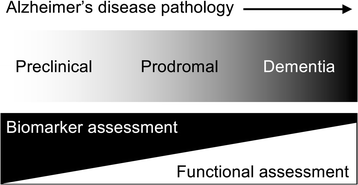
Context: This commentary discusses the implications of disease-modifying treatments for Alzheimer's disease which seem likely to appear in the next few years and results from a meeting of British experts in neurodegenerative diseases in Edinburgh. The availability of such treatments would help change public and professional attitudes and accelerate engagement with the prodromal and preclinical populations who might benefit from them. However, this would require an updated understanding of Alzheimer's disease, namely the important distinction between Alzheimer's disease and Alzheimer's dementia.
Consensus: Since treatments are likely to be most effective in the early stages, identification of clinically relevant brain changes (for example, amyloid burden using imaging or cerebrospinal fluid biomarkers) will be crucial. While current biomarkers could be useful in identifying eligibility for new therapies, trial data are not available to aid decisions about stopping or continuing treatment in clinical practice. Therefore, effective monitoring of safety and effectiveness when these treatments are introduced into clinical practice will be necessary to inform wide-scale use. Equity of access is key but there is a tension between universal access for everyone with a diagnosis of Alzheimer's disease and specifying an eligible population most likely to respond. We propose the resources necessary for an optimal care pathway as well as the necessary education and training for primary and secondary care.
Conclusion: The majority of current services in the UK and elsewhere would not be able to accommodate the specialist investigations required to select patients and prescribe these therapies. Therefore, a stepped approach would be necessary: from innovating sentinel clinical-academic centres that already have capacity to deliver the necessary phase IV trials, through early adoption in a hub and spoke model, to nationwide adoption for true equity of access. The optimism generated by recent and anticipated developments in the understanding and treatment of Alzheimer's disease presents a great opportunity to innovate and adapt our services to incorporate the next exciting development in the field of dementia.
Keywords: Alzheimer’s disease; Clinical trials; Dementia; Disease-modification; Service redesign; Therapeutics.
Conflict of interest statement
Authors’ information
The authors’ main or most relevant affiliation has been recorded.
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have seen and approved the submitted version of the manuscript.
Competing interests
Bob Barber has participated in an advisory panel for Novartis on one occasion and conducted a number of pharmaceutical sponsored commercial clinical trials in Alzheimer’s disease (including Lilly, Roche and Janssen). He has a national role for NIHR NHS relating to commercial studies in dementia. Nick Fox has received research support from AVID/Lilly and has advised GSK, Lilly, Novartis and Roche; he serves on a data monitoring committee for Biogen—all payments for these activities are to UCL. Clive Holmes has attended advisory boards for Lilly pharmaceuticals. Martin Rossor serves on a safety monitoring committee for Servier and has advised Merck on patient registries. Tom Russ works within NHS Scotland with the Scottish Neuroprogressive and Dementia Network on commercial studies in dementia. All authors have completed the ICJME declaration of conflicts of interest and these are available on request.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- World Health Organization . Alzheimer’s Disease International: Dementia: a public health priority. Geneva: World Health Organization; 2012.
-
- Prince M, Guerchet M, Prina M. Alzheimer’s Disease International: Policy brief for heads of government: the global impact of dementia 2013–2050. London: Alzheimer Disease International; 2013.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

