The Kölliker-Fuse nucleus orchestrates the timing of expiratory abdominal nerve bursting
- PMID: 29070631
- PMCID: PMC5867379
- DOI: 10.1152/jn.00499.2017
The Kölliker-Fuse nucleus orchestrates the timing of expiratory abdominal nerve bursting
Abstract
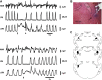
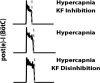
Coordination of respiratory pump and valve muscle activity is essential for normal breathing. A hallmark respiratory response to hypercapnia and hypoxia is the emergence of active exhalation, characterized by abdominal muscle pumping during the late one-third of expiration (late-E phase). Late-E abdominal activity during hypercapnia has been attributed to the activation of expiratory neurons located within the parafacial respiratory group (pFRG). However, the mechanisms that control emergence of active exhalation, and its silencing in restful breathing, are not completely understood. We hypothesized that inputs from the Kölliker-Fuse nucleus (KF) control the emergence of late-E activity during hypercapnia. Previously, we reported that reversible inhibition of the KF reduced postinspiratory (post-I) motor output to laryngeal adductor muscles and brought forward the onset of hypercapnia-induced late-E abdominal activity. Here we explored the contribution of the KF for late-E abdominal recruitment during hypercapnia by pharmacologically disinhibiting the KF in in situ decerebrate arterially perfused rat preparations. These data were combined with previous results and incorporated into a computational model of the respiratory central pattern generator. Disinhibition of the KF through local parenchymal microinjections of gabazine (GABAA receptor antagonist) prolonged vagal post-I activity and inhibited late-E abdominal output during hypercapnia. In silico, we reproduced this behavior and predicted a mechanism in which the KF provides excitatory drive to post-I inhibitory neurons, which in turn inhibit late-E neurons of the pFRG. Although the exact mechanism proposed by the model requires testing, our data confirm that the KF modulates the formation of late-E abdominal activity during hypercapnia. NEW & NOTEWORTHY The pons is essential for the formation of the three-phase respiratory pattern, controlling the inspiratory-expiratory phase transition. We provide functional evidence of a novel role for the Kölliker-Fuse nucleus (KF) controlling the emergence of abdominal expiratory bursts during active expiration. A computational model of the respiratory central pattern generator predicts a possible mechanism by which the KF interacts indirectly with the parafacial respiratory group and exerts an inhibitory effect on the expiratory conditional oscillator.
Keywords: abdominal expiratory activity; active expiration; pons; respiratory pattern; ventral respiratory column.
Figures








References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

