Minocycline modulates microglia polarization in ischemia-reperfusion model of retinal degeneration and induces neuroprotection
- PMID: 29070819
- PMCID: PMC5656679
- DOI: 10.1038/s41598-017-14450-5
Minocycline modulates microglia polarization in ischemia-reperfusion model of retinal degeneration and induces neuroprotection
Abstract
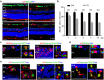
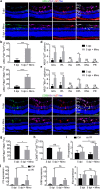
Retinal ischemia-reperfusion (IR) injury causes irreversible loss of neurons and ultimately leads to permanent visual impairment and blindness. The cellular response under this pathological retinal condition is less clear. Using genetically modified mice, we systematically examined the behavior of microglia/macrophages after injury. We show that IR leads to activation of microglia/macrophages indicated by migration and proliferation of resident microglia and recruitment of circulating monocytes. IR-induced microglia/macrophages associate with apoptotic retinal neurons. Very interestingly, neuron loss can be mitigated by minocycline treatment. Minocycline induces Il4 expression and M2 polarization of microglia/macrophages. IL4 neutralization dampens minocycline-induced M2 polarization and neuroprotection. Given a well-established safety profile as an antibiotic, our results provide a rationale for using minocycline as a therapeutic agent for treating ischemic retinal degeneration.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






Similar articles
-
Carbon monoxide treatment reduces microglial activation in the ischemic rat retina.Graefes Arch Clin Exp Ophthalmol. 2016 Oct;254(10):1967-1976. doi: 10.1007/s00417-016-3435-6. Epub 2016 Jul 21. Graefes Arch Clin Exp Ophthalmol. 2016. PMID: 27443355
-
Low-dose minocycline mediated neuroprotection on retinal ischemia-reperfusion injury of mice.Mol Vis. 2018 May 18;24:367-378. eCollection 2018. Mol Vis. 2018. PMID: 29853771 Free PMC article.
-
Minocycline suppresses disease-associated microglia (DAM) in a model of photoreceptor cell degeneration.Exp Eye Res. 2022 Apr;217:108953. doi: 10.1016/j.exer.2022.108953. Epub 2022 Jan 25. Exp Eye Res. 2022. PMID: 35090890
-
The role of minocycline in ischemia-reperfusion injury: a comprehensive review of an old drug with new implications.Recent Pat Cardiovasc Drug Discov. 2011 May;6(2):123-32. doi: 10.2174/157489011795933783. Recent Pat Cardiovasc Drug Discov. 2011. PMID: 21599619 Review.
-
Non-analgesic effects of opioids: neuroprotection in the retina.Curr Pharm Des. 2012;18(37):6101-8. doi: 10.2174/138161212803582441. Curr Pharm Des. 2012. PMID: 22747547 Review.
Cited by
-
Neuroprotective Effect of Fractalkine on Radiation-induced Brain Injury Through Promoting the M2 Polarization of Microglia.Mol Neurobiol. 2021 Mar;58(3):1074-1087. doi: 10.1007/s12035-020-02138-3. Epub 2020 Oct 22. Mol Neurobiol. 2021. PMID: 33089423 Free PMC article.
-
Therapeutic Targeting of Retinal Immune Microenvironment With CSF-1 Receptor Antibody Promotes Visual Function Recovery After Ischemic Optic Neuropathy.Front Immunol. 2020 Nov 13;11:585918. doi: 10.3389/fimmu.2020.585918. eCollection 2020. Front Immunol. 2020. PMID: 33281816 Free PMC article.
-
The effects of a highly bioavailable curcumin PhytosomeTM preparation on the retinal architecture and glial reactivity in the GFAP-IL6 mice.Front Ophthalmol (Lausanne). 2023 Oct 6;3:1205542. doi: 10.3389/fopht.2023.1205542. eCollection 2023. Front Ophthalmol (Lausanne). 2023. PMID: 38983084 Free PMC article.
-
Betaine Inhibits NLRP3 Inflammasome Hyperactivation and Regulates Microglial M1/M2 Phenotypic Differentiation, Thereby Attenuating Lipopolysaccharide-Induced Depression-Like Behavior.J Immunol Res. 2022 Oct 26;2022:9313436. doi: 10.1155/2022/9313436. eCollection 2022. J Immunol Res. 2022. PMID: 36339940 Free PMC article.
-
Minocycline Inhibits Microglial Activation and Improves Visual Function in a Chronic Model of Age-Related Retinal Degeneration.Biomedicines. 2022 Dec 12;10(12):3222. doi: 10.3390/biomedicines10123222. Biomedicines. 2022. PMID: 36551980 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

