Melanopsin, a Canonical Light Receptor, Mediates Thermal Activation of Clock Genes
- PMID: 29070825
- PMCID: PMC5656685
- DOI: 10.1038/s41598-017-13939-3
Melanopsin, a Canonical Light Receptor, Mediates Thermal Activation of Clock Genes
Abstract
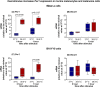
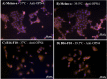
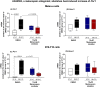
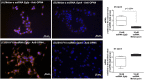
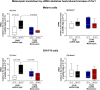
Melanopsin (OPN4) is a photo-pigment found in a small subset of intrinsically photosensitive ganglion cells (ipRGCs) of the mammalian retina. These cells play a role in synchronizing the central circadian pacemaker to the astronomical day by conveying information about ambient light to the hypothalamic suprachiasmatic nucleus, the site of the master clock. We evaluated the effect of a heat stimulus (39.5 °C) on clock gene (Per1 and Bmal1) expression in cultured murine Melan-a melanocytes synchronized by medium changes, and in B16-F10 melanoma cells, in the presence of the selective OPN4 antagonist AA92593, or after OPN4 knockdown by small interfering RNA (siRNA). In addition, we evaluated the effects of heat shock on the localization of melanopsin by immunocytochemistry. In both cell lines melanopsin was found in a region capping the nucleus and heat shock did not affect its location. The heat-induced increase of Per1 expression was inhibited when melanopsin was pharmacologically blocked by AA92593 as well as when its protein expression was suppressed by siRNA in both Melan-a and B16-F10 cells. These data strongly suggest that melanopsin is required for thermo-reception, acting as a thermo-opsin that ultimately feeds the local circadian clock in mouse melanocytes and melanoma cells.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
The effect of white light on normal and malignant murine melanocytes: A link between opsins, clock genes, and melanogenesis.Biochim Biophys Acta. 2016 Jun;1863(6 Pt A):1119-33. doi: 10.1016/j.bbamcr.2016.03.001. Epub 2016 Mar 3. Biochim Biophys Acta. 2016. PMID: 26947915
-
Intrinsically Photosensitive Retinal Ganglion Cells (ipRGCs) Are Necessary for Light Entrainment of Peripheral Clocks.PLoS One. 2016 Dec 16;11(12):e0168651. doi: 10.1371/journal.pone.0168651. eCollection 2016. PLoS One. 2016. PMID: 27992553 Free PMC article.
-
From blue light to clock genes in zebrafish ZEM-2S cells.PLoS One. 2014 Sep 3;9(9):e106252. doi: 10.1371/journal.pone.0106252. eCollection 2014. PLoS One. 2014. PMID: 25184495 Free PMC article.
-
How rod, cone, and melanopsin photoreceptors come together to enlighten the mammalian circadian clock.Prog Brain Res. 2012;199:1-18. doi: 10.1016/B978-0-444-59427-3.00001-0. Prog Brain Res. 2012. PMID: 22877656 Review.
-
Genetic advances in ophthalmology: the role of melanopsin-expressing, intrinsically photosensitive retinal ganglion cells in the circadian organization of the visual system.Semin Ophthalmol. 2013 Sep-Nov;28(5-6):406-21. doi: 10.3109/08820538.2013.825294. Epub 2013 Sep 6. Semin Ophthalmol. 2013. PMID: 24010846 Review.
Cited by
-
Expression of the Circadian Clock Gene BMAL1 Positively Correlates With Antitumor Immunity and Patient Survival in Metastatic Melanoma.Front Oncol. 2018 Jun 12;8:185. doi: 10.3389/fonc.2018.00185. eCollection 2018. Front Oncol. 2018. PMID: 29946530 Free PMC article.
-
The circadian clock and metabolic homeostasis: entangled networks.Cell Mol Life Sci. 2021 May;78(10):4563-4587. doi: 10.1007/s00018-021-03800-2. Epub 2021 Mar 8. Cell Mol Life Sci. 2021. PMID: 33683376 Free PMC article. Review.
-
Melanopsin (Opn4) is an oncogene in cutaneous melanoma.Commun Biol. 2022 May 13;5(1):461. doi: 10.1038/s42003-022-03425-6. Commun Biol. 2022. PMID: 35562405 Free PMC article.
-
Recognition of Melanocytes in Immuno-Neuroendocrinology and Circadian Rhythms: Beyond the Conventional Melanin Synthesis.Cells. 2022 Jun 30;11(13):2082. doi: 10.3390/cells11132082. Cells. 2022. PMID: 35805166 Free PMC article. Review.
-
Non-Metastatic Cutaneous Melanoma Induces Chronodisruption in Central and Peripheral Circadian Clocks.Int J Mol Sci. 2018 Apr 3;19(4):1065. doi: 10.3390/ijms19041065. Int J Mol Sci. 2018. PMID: 29614021 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

