Dual negative roles of C/EBPα in the expansion and pro-tumor functions of MDSCs
- PMID: 29070836
- PMCID: PMC5656646
- DOI: 10.1038/s41598-017-12968-2
Dual negative roles of C/EBPα in the expansion and pro-tumor functions of MDSCs
Abstract
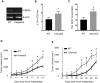
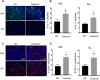
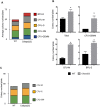
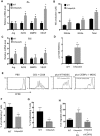
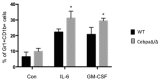
Myeloid-derived suppressor cells (MDSCs) are greatly expanded in cancer patients and tumor-bearing mice. They infiltrate into tumors and modulate the tumor microenvironment. In an effort to identify molecular mediators responsible for expansion and the tumor-promoting function of MDSCs, we discovered CCAAT/enhancer binding protein alpha (C/EBPα) expression was significantly reduced in MDSCs from tumor-bearing mice compared to non-tumor-bearing hosts. Tumor-conditioned medium down-regulated C/EBPα expression, suggesting tumor secreted factors inhibiting the gene expression. Consistent with the function of C/EBPα in regulating the balance between proliferation and growth arrest in hematopoietic progenitors, myeloid lineage specific deletion of C/EBPα resulted in significantly enhanced MDSC proliferation and expansion, as well as an increase of myeloid progenitors and a decrease of mature cells. In addition, deletion of C/EBPα in MDSCs enhanced the pro-angiogenic, immune suppressive and pro-tumorigenic behavior of these cells by upregulating the production of iNOS and arginase, as well as MMP-9 and VEGF. Accordingly, tumors growing in C/EBPα conditional null mice displayed greater MDSC infiltration, increased vascularization and accelerated tumor growth. Taken together, this study reveals dual negative roles of C/EBPα in the expansion as well as pro-angiogenic and immune suppressive functions in MDSCs.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

