Characterization of histone acylations links chromatin modifications with metabolism
- PMID: 29070843
- PMCID: PMC5656686
- DOI: 10.1038/s41467-017-01384-9
Characterization of histone acylations links chromatin modifications with metabolism
Abstract
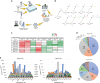
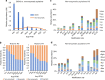
Over the last decade, numerous histone acyl post-translational modifications (acyl-PTMs) have been discovered, of which the functional significance is still under intense study. Here, we use high-resolution mass spectrometry to accurately quantify eight acyl-PTMs in vivo and after in vitro enzymatic assays. We assess the ability of seven histone acetyltransferases (HATs) to catalyze acylations on histones in vitro using short-chain acyl-CoA donors, proving that they are less efficient towards larger acyl-CoAs. We also observe that acyl-CoAs can acylate histones through non-enzymatic mechanisms. Using integrated metabolomic and proteomic approaches, we achieve high correlation (R 2 > 0.99) between the abundance of acyl-CoAs and their corresponding acyl-PTMs. Moreover, we observe a dose-dependent increase in histone acyl-PTM abundances in response to acyl-CoA supplementation in in nucleo reactions. This study represents a comprehensive profiling of scarcely investigated low-abundance histone marks, revealing that concentrations of acyl-CoAs affect histone acyl-PTM abundances by both enzymatic and non-enzymatic mechanisms.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
Publication types
Grants and funding
- R35 GM118090/GM/NIGMS NIH HHS/United States
- R01 GM101664/GM/NIGMS NIH HHS/United States
- R01 GM100907/GM/NIGMS NIH HHS/United States
- P01 CA196539/CA/NCI NIH HHS/United States
- P01 AG031862/AG/NIA NIH HHS/United States
- P30 ES013508/ES/NIEHS NIH HHS/United States
- R21 HD087866/HD/NICHD NIH HHS/United States
- R03 CA211820/CA/NCI NIH HHS/United States
- R01 GM060293/GM/NIGMS NIH HHS/United States
- T32 GM008275/GM/NIGMS NIH HHS/United States
- K22 ES026235/ES/NIEHS NIH HHS/United States
- R01 AI118891/AI/NIAID NIH HHS/United States
- R01 GM110174/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

