Effect of adipose-derived mesenchymal stem cell transplantation on vascular calcification in rats with adenine-induced kidney disease
- PMID: 29070880
- PMCID: PMC5656613
- DOI: 10.1038/s41598-017-14492-9
Effect of adipose-derived mesenchymal stem cell transplantation on vascular calcification in rats with adenine-induced kidney disease
Abstract
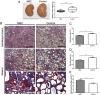
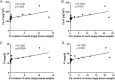
Previous studies have investigated the use of mesenchymal stem cells (MSCs) to treat damaged kidneys. However, the effect of adipose-derived MSCs (ASCs) on vascular calcification in chronic kidney disease (CKD) is still poorly understood. In the present study, we explored the potential of ASCs for the treatment of CKD and vascular calcification. CKD was induced in male Sprague-Dawley rats by feeding them a diet containing 0.75% adenine for 4 weeks. ASCs transplantation significantly reduced serum inorganic phosphorus (Pi) as compared to that in the control. The histopathology of the kidneys showed a greater dilation of tubular lumens and interstitial fibrosis in the control group. Calcium and Pi contents of the aorta in the ASCs transplantation group were lower than those in the control group. Von Kossa staining of the thoracic aorta media revealed that ASCs transplantation suppressed vascular calcification. Thus, this study revealed that autogenic ASCs transplantation inhibits kidney damage and suppresses the progression of vascular calcification in the CKD rat model, suggesting that autogenic ASCs transplantation is a novel approach for preventing the progression of CKD and vascular calcification.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






Similar articles
-
Metanephros transplantation inhibits the progression of vascular calcification in rats with adenine-induced renal failure.Nephron Exp Nephrol. 2012;120(1):e32-40. doi: 10.1159/000332012. Epub 2011 Dec 23. Nephron Exp Nephrol. 2012. PMID: 22205150
-
Osteogenesis of heterotopically transplanted mesenchymal stromal cells in rat models of chronic kidney disease.J Bone Miner Res. 2013 Dec;28(12):2523-34. doi: 10.1002/jbmr.1994. J Bone Miner Res. 2013. PMID: 23703894
-
Effect of cross-linked chitosan iron (III) on vascular calcification in uremic rats.Exp Biol Med (Maywood). 2018 May;243(9):796-802. doi: 10.1177/1535370218775035. Exp Biol Med (Maywood). 2018. PMID: 29763365 Free PMC article.
-
Adenine-induced chronic kidney disease in rats.Nephrology (Carlton). 2018 Jan;23(1):5-11. doi: 10.1111/nep.13180. Nephrology (Carlton). 2018. PMID: 29030945 Review.
-
Vascular calcification in animal models of CKD: A review.Am J Nephrol. 2010;31(6):471-81. doi: 10.1159/000299794. Epub 2010 Apr 22. Am J Nephrol. 2010. PMID: 20413965 Review.
Cited by
-
Treatment effects of human amnion-derived mesenchymal stem cells for skin lesions and metastatic pulmonary calcification in calciphylaxis patients - case series and literature review.Ren Fail. 2025 Dec;47(1):2516207. doi: 10.1080/0886022X.2025.2516207. Epub 2025 Jun 15. Ren Fail. 2025. PMID: 40518559 Free PMC article. Review.
-
Reno-protection of Urine-derived Stem Cells in A Chronic Kidney Disease Rat Model Induced by Renal Ischemia and Nephrotoxicity.Int J Biol Sci. 2020 Jan 1;16(3):435-446. doi: 10.7150/ijbs.37550. eCollection 2020. Int J Biol Sci. 2020. PMID: 32015680 Free PMC article.
-
Mesenchymal stem cell treatment for enteric neuropathy in the Winnie mouse model of spontaneous chronic colitis.Cell Tissue Res. 2022 Jul;389(1):41-70. doi: 10.1007/s00441-022-03633-w. Epub 2022 May 10. Cell Tissue Res. 2022. PMID: 35536444
-
Exosomes Derived From Mesenchymal Stromal Cells Pretreated With Advanced Glycation End Product-Bovine Serum Albumin Inhibit Calcification of Vascular Smooth Muscle Cells.Front Endocrinol (Lausanne). 2018 Sep 21;9:524. doi: 10.3389/fendo.2018.00524. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 30298051 Free PMC article.
-
Mesenchymal stem cells ameliorate renal fibrosis by galectin-3/Akt/GSK3β/Snail signaling pathway in adenine-induced nephropathy rat.Stem Cell Res Ther. 2021 Jul 16;12(1):409. doi: 10.1186/s13287-021-02429-z. Stem Cell Res Ther. 2021. Retraction in: Stem Cell Res Ther. 2024 Feb 27;15(1):52. doi: 10.1186/s13287-024-03668-6. PMID: 34271976 Free PMC article. Retracted.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

