Silodosin in the treatment of distal ureteric stones in children: A prospective, randomised, placebo-controlled study
- PMID: 29071151
- PMCID: PMC5651944
- DOI: 10.1016/j.aju.2017.05.005
Silodosin in the treatment of distal ureteric stones in children: A prospective, randomised, placebo-controlled study
Abstract
Objectives: To evaluate the possible role of silodosin (a highly selective α1A-adrenoceptor antagonist) in facilitating the passage of distal ureteric stones (DUS) in children, as the role of α-blockers as medical expulsive therapy is well known in adults.
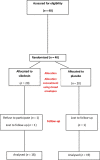
Patients and methods: In all, 40 paediatric patients (27 boys and 13 girls) diagnosed with unilateral, single, radiopaque DUS of <10 mm were included in the study. Their mean (SD, range) age was 8.1 (2.7, 5-17) years. The patients were randomly divided into two groups: Group A, received silodosin 4 mg as a single bedtime dose; and Group B, received placebo as a single bedtime dose. Ibuprofen was prescribed to both groups on-demand for pain episode relief. Patients were followed up biweekly for 4 weeks. The stone expulsion time and rate, pain episodes, analgesic use, and any adverse effects were recorded.
Results: The mean (SD) stone size in Group A was 6.6 (1.7) mm and in Group B was 6.7 (1.4) mm (P = 0.4). Two patients were lost to follow-up (one from each group), and one patient in Group A refused to complete the study. The stone-free rate at end of the 4-week treatment period was 88.8% in Group A vs 73.6% in Group B (P = 0.4). The mean (SD) stone expulsion time was 7.0 (4.3) vs 10.4 (4.7) days in groups A and B, respectively (P = 0.02). The mean (SD) number of pain episodes requiring ibuprofen was 2.3 (1.4) vs 4.7 (2.6) episodes in groups A and B, respectively (P < 0.001). Adverse effects (headache and dizziness) were recorded in three patients (16.7%) in Group A, which were mild and none of them discontinued treatment, whilst no adverse effects were recorded in Group B.
Conclusions: The data in the present study show that silodosin can be safely used in the treatment of DUS in children for decreasing time to stone expulsion, pain episodes, and analgesic requirement.
Keywords: Children; Distal ureteric stone (DUS); KUB, plain abdominal radiograph of the kidneys, ureters and bladder; MET, medical expulsive therapy; Medical expulsive therapy; SFR, stone-free rate; SWL, shockwave lithotripsy; Silodosin; URS, ureteroscopy.
References
-
- Kroovand R.L. Pediatric urolithiasis. Urol Clin North Am. 1997;24:173–184. - PubMed
-
- Milliner D.S., Murphy M.E. Urolithiasis in pediatric patients. Mayo Clin Proc. 1993;68:241–248. - PubMed
-
- García-Nieto V., Siverio B., Monge M., Toledo C., Molini N. Urinary calcium excretion in children with vesicoureteral reflux. Nephrol Dial Transplant. 2003;18:507–511. - PubMed
-
- Hesse A., Siener R. Current aspects of epidemiology and nutrition in urinary stone disease. World J Urol. 1997;15:165–171. - PubMed
-
- Beach M.A., Mauro L.S. Pharmacologic expulsive treatment of ureteral calculi. Ann Pharmacother. 2006;40:1361–1368. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous