Heparin-Regulated Prodrug-Type Macromolecular Theranostic Systems for Cancer Therapy
- PMID: 29071181
- PMCID: PMC5646728
- DOI: 10.7150/ntno.18292
Heparin-Regulated Prodrug-Type Macromolecular Theranostic Systems for Cancer Therapy
Abstract
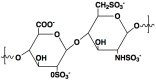
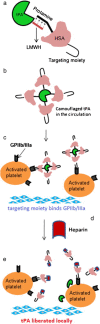
Heparin is a kind of naturally occurring polymer with excellent biocompatibility and solubility. It is characterized by dense of negative charge, higher than any endogenous components. Heparin can bind with various cationic peptides and proteins, thereby providing a useful noncovalent linkage for building a drug delivery system. As a case in point, heparin/cell-penetrating peptides (CPP) interaction is strong, and remains stable in vivo. They can be used to modify different proteins, respectively, and subsequently, by simply mixing the modified proteins, a protein-protein conjugate can be form via the stable heparin/CPP linkage. This linkage could not be broken unless addition of protamine that bears higher cationic charge density than CPP, and CPP thus can be substituted and released. Of note, heparin is a potent antagonist of CPP, and their binding naturally inhibits CPP-mediated drug cell penetration. Based on this method, we developed a heparin-regulated macromolecular prodrug-type system, termed ATTEMPTS, for drug targeting delivery. In this review article, we mainly summary the application of ATTEMPTS in delivery of various macromolecular drugs for cancer therapy, and also introduce the heparin-regulated nanoprobes for tumor imaging.
Keywords: Heparin; drug targeting delivery; heparin-regulated macromolecular prodrug-type system.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




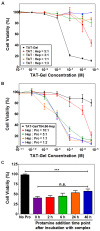
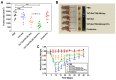
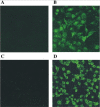
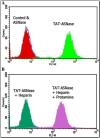




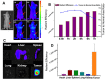
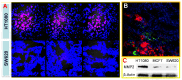
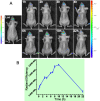

Similar articles
-
15 years of ATTEMPTS: a macromolecular drug delivery system based on the CPP-mediated intracellular drug delivery and antibody targeting.J Control Release. 2015 May 10;205:58-69. doi: 10.1016/j.jconrel.2014.12.002. Epub 2014 Dec 4. J Control Release. 2015. PMID: 25483423 Review.
-
ATTEMPTS system: a macromolecular prodrug strategy for cancer drug delivery.Curr Pharm Des. 2010 Jul;16(21):2369-76. doi: 10.2174/138161210791920441. Curr Pharm Des. 2010. PMID: 20618157 Review.
-
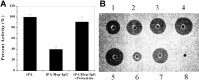
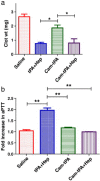
Construction and characterization of a t-PA mutant for use in ATTEMPTS: a drug delivery system for achieving targeted thrombolysis.J Control Release. 2005 Dec 10;110(1):164-76. doi: 10.1016/j.jconrel.2005.09.027. Epub 2005 Nov 2. J Control Release. 2005. PMID: 16260060
-
ATTEMPTS: a heparin/protamine-based triggered release system for the delivery of enzyme drugs without associated side-effects.Adv Drug Deliv Rev. 2003 Feb 10;55(2):251-65. doi: 10.1016/s0169-409x(02)00181-3. Adv Drug Deliv Rev. 2003. PMID: 12564979 Review.
-
PTD-Modified ATTEMPTS for Enhanced Toxin-based Cancer Therapy: An In Vivo Proof-of-Concept Study.Pharm Res. 2015 Aug;32(8):2690-703. doi: 10.1007/s11095-015-1653-y. Epub 2015 Feb 21. Pharm Res. 2015. PMID: 25701313 Free PMC article.
Cited by
-
Polymer-drug conjugates: revolutionizing nanotheranostic agents for diagnosis and therapy.Discov Oncol. 2024 Nov 11;15(1):641. doi: 10.1007/s12672-024-01509-9. Discov Oncol. 2024. PMID: 39527173 Free PMC article. Review.
-
Advances on Delivery of Cytotoxic Enzymes as Anticancer Agents.Molecules. 2022 Jun 14;27(12):3836. doi: 10.3390/molecules27123836. Molecules. 2022. PMID: 35744957 Free PMC article. Review.
-
Heparin, Heparin-like Molecules, and Heparin Mimetics in Breast Cancer: A Concise Review.Biomolecules. 2025 Jul 17;15(7):1034. doi: 10.3390/biom15071034. Biomolecules. 2025. PMID: 40723905 Free PMC article. Review.
-
Advances on Tumor-Targeting Delivery of Cytotoxic Proteins.ACS Pharmacol Transl Sci. 2019 Dec 30;3(1):107-118. doi: 10.1021/acsptsci.9b00087. eCollection 2020 Feb 14. ACS Pharmacol Transl Sci. 2019. PMID: 32259092 Free PMC article. Review.
-
Cell-Penetrating Peptides Enhance the Activity of Human Fibroblast Growth Factor 2 by Prolonging the Retention Time: A New Vision for Drug-Delivery Systems.Int J Mol Sci. 2020 Jan 10;21(2):442. doi: 10.3390/ijms21020442. Int J Mol Sci. 2020. PMID: 32284513 Free PMC article.
References
-
- Lee YC, Lee RT. Carbohydrate-Protein Interactions - Basis of Glycobiology. Accounts of Chemical Research. 1995;28:321–7.
-
- Powell AK, Yates EA, Fernig DG, Turnbull JE. Interactions of heparin/heparan sulfate with proteins: appraisal of structural factors and experimental approaches. Glycobiology. 2004;14:17R–30R. - PubMed
-
- Capila I, Linhardt RJ. Heparin-protein interactions. Angew Chem Int Ed Engl. 2002;41:391–412. - PubMed
-
- George CM. Thromboembolic Disease. In: Helms RA, Quan DJ, editors. Textbook of therapeutics: drug and disease management. 8th ed. Philadelphia: Lippincott Williams & Wilkins; 2006. p. 634.
-
- Mecca T, Consoli GM, Geraci C, La Spina R, Cunsolo F. Polycationic calix[8]arenes able to recognize and neutralize heparin. Org Biomol Chem. 2006;4:3763–8. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

