Prediction of Anti-cancer Nanotherapy Efficacy by Imaging
- PMID: 29071194
- PMCID: PMC5646731
- DOI: 10.7150/ntno.20564
Prediction of Anti-cancer Nanotherapy Efficacy by Imaging
Abstract
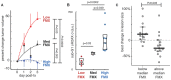
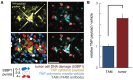
Anticancer nanotherapeutics have shown mixed results in clinical trials, raising the questions of whether imaging should be used to i) identify patients with a higher likelihood of nanoparticle accumulation, ii) assess nanotherapeutic efficacy before traditional measures show response, and iii) guide adjuvant treatments to enhance therapeutic nanoparticle (TNP) delivery. Here we review the use of a clinically approved MRI nanoparticle (ferumoxytol, FMX) to predict TNP delivery and efficacy. It is becoming increasingly apparent that nanoparticles used for imaging, despite clearly distinct physicochemical properties, often co-localize with TNP in tumors. This evidence offers the possibility of using FMX as a generic "companion diagnostic" nanoparticle for multiple TNP formulations, thus potentially allowing many of the complex regulatory and cost challenges of other approaches to be avoided.
Keywords: dextran-coated iron oxide nanoparticle; enhanced permeability and retention effect; magnetic resonance imaging; nanomedicine; personalized medicine; tumor associated macrophage..
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

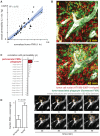
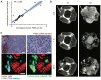
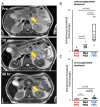
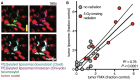
References
-
- Juliano RL, Stamp D. Pharmacokinetics of liposome-encapsulated anti-tumor drugs. Studies with vinblastine, actinomycin D, cytosine arabinoside, and daunomycin. Biochem Pharmacol. 1978;27:21–7. - PubMed
-
- Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol Rev. 2001;53:283–318. - PubMed
-
- O'Brien ME, Wigler N, Inbar M, Rosso R, Grischke E, Santoro A. et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol. 2004;15:440–9. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

