Transforming growth factor-β enhances Rho-kinase activity and contraction in airway smooth muscle via the nucleotide exchange factor ARHGEF1
- PMID: 29071730
- PMCID: PMC5746525
- DOI: 10.1113/JP275033
Transforming growth factor-β enhances Rho-kinase activity and contraction in airway smooth muscle via the nucleotide exchange factor ARHGEF1
Abstract
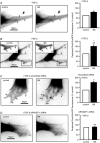
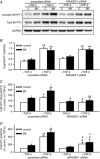
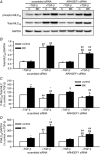
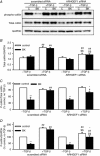
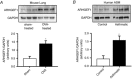
Key points: Transforming growth-factor-β (TGF-β) and RhoA/Rho-kinase are independently implicated in the airway hyper-responsiveness associated with asthma, but how these proteins interact is not fully understood. We examined the effects of pre-treatment with TGF-β on expression and activity of RhoA, Rho-kinase and ARHGEF1, an activator of RhoA, as well as on bradykinin-induced contraction, in airway smooth muscle. TGF-β enhanced bradykinin-induced RhoA translocation, Rho-kinase-dependent phosphorylation and contraction, but partially suppressed bradykinin-induced RhoA activity (RhoA-GTP content). TGF-β enhanced the expression of ARHGEF1, while a small interfering RNA against ARHGEF1 and a RhoGEF inhibitor prevented the effects of TGF-β on RhoA and Rho-kinase activity and contraction, respectively. ARHGEF1 expression was also enhanced in airway smooth muscle from asthmatic patients and ovalbumin-sensitized mice. ARHGEF1 is a key TGF-β target gene, an important regulator of Rho-kinase activity and therefore a potential therapeutic target for the treatment of asthmatic airway hyper-responsiveness.
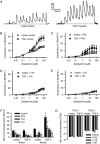
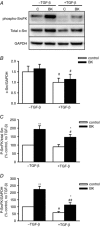
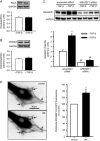
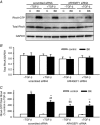
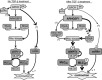
Abstract: Transforming growth factor-β (TGF-β), RhoA/Rho-kinase and Src-family kinases (SrcFK) have independently been implicated in airway hyper-responsiveness, but how they interact to regulate airway smooth muscle contractility is not fully understood. We found that TGF-β pre-treatment enhanced acute contractile responses to bradykinin (BK) in isolated rat bronchioles, and inhibitors of RhoGEFs (Y16) and Rho-kinase (Y27632), but not the SrcFK inhibitor PP2, prevented this enhancement. In cultured human airway smooth muscle cells (hASMCs), TGF-β pre-treatment enhanced the protein expression of the Rho guanine nucleotide exchange factor ARHGEF1, MLC20 , MYPT-1 and the actin-severing protein cofilin, but not of RhoA, ROCK2 or c-Src. In hASMCs, acute treatment with BK triggered subcellular translocation of ARHGEF1 and RhoA and enhanced auto-phosphorylation of SrcFK and phosphorylation of MYPT1 and MLC20 , but induced de-phosphorylation of cofilin. TGF-β pre-treatment amplified the effects of BK on RhoA translocation and MYPT1/MLC20 phosphorylation, but suppressed the effects of BK on RhoA-GTP content, SrcFK auto-phosphorylation and cofilin de-phosphorylation. In hASMCs, an ARHGEF1 small interfering RNA suppressed the effects of BK and TGF-β on RhoA-GTP content, RhoA translocation and MYPT1 and MLC20 phosphorylation, but minimally influenced the effects of TGF-β on cofilin expression and phosphorylation. ARHGEF1 expression was also enhanced in ASMCs of asthmatic patients and in lungs of ovalbumin-sensitized mice. Our data indicate that TGF-β enhances BK-induced contraction, RhoA translocation and Rho-kinase activity in airway smooth muscle largely via ARHGEF1, but independently of SrcFK and total RhoA-GTP content. A role for smooth muscle ARHGEF1 in asthmatic airway hyper-responsiveness is worthy of further investigation.
Keywords: Rho-kinase; TGF-β; airway smooth muscle.
© 2017 The Authors. The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Figures
References
-
- Albinsson S, Nordstrom I & Hellstrand P (2004). Stretch of the vascular wall induces smooth muscle differentiation by promoting actin polymerization. J Biol Chem 279, 34849–34855. - PubMed
-
- Andrianantoandro E & Pollard TD (2006). Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/cofilin. Mol Cell 24, 13–23. - PubMed
-
- Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O & Caroni P (1998). Regulation of actin dynamics through phosphorylation of cofilin by LIM‐kinase. Nature 393, 805–809. - PubMed
-
- Atfi A, Drobetsky E, Boissonneault M, Chapdelaine A & Chevalier S (1994). Transforming growth factor β down‐regulates Src family protein tyrosine kinase signaling pathways. J Biol Chem 269, 30688–30693. - PubMed
-
- Birmingham A, Anderson E, Sullivan K, Reynolds A, Boese Q, Leake D, Karpilow J & Khvorova A (2007). A protocol for designing siRNAs with high functionality and specificity. Nat Protoc 2, 2068–2078. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

