Effects of a lipid emulsion containing fish oil on polyunsaturated fatty acid profiles, growth and morbidities in extremely premature infants: A randomized controlled trial
- PMID: 29072164
- PMCID: PMC5784264
- DOI: 10.1016/j.clnesp.2017.04.004
Effects of a lipid emulsion containing fish oil on polyunsaturated fatty acid profiles, growth and morbidities in extremely premature infants: A randomized controlled trial
Abstract
Background & aims: The purpose of the study was to compare the effects of the parenteral emulsion SMOFlipid®, with 15% fish oil, with Clinoleic® on retinopathy of prematurity (ROP) and other morbidities and growth, and to compare their impact on longitudinal serum levels of fatty acids. Retinopathy of prematurity, other morbidity and growth were correlated with each parenteral lipid supplement.
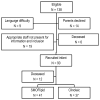
Methods: Ninety infants born at gestational age <28 weeks were randomized to treatment with SMOFlipid® or Clinoleic®. Two thirds (66%) of the infants received parenteral nutrition for up to 14 days birth (median 8, range 2-14 days), and additional 25% of the infants received for up to 28 days after birth (median 21, range 15-28 days). Cord blood samples and then venous blood samples were obtained at ages 1, 7, 14, and 28 days and at postmenstrual age (PMA) 32, 36, and 40 weeks. Breastmilk was collected at postnatal day 7, and at PMA 32 and 40 weeks. Serum phospholipid and breastmilk total fatty acids were analyzed by gas chromatography-mass spectrometry. Treatment groups were compared with regard to ROP, bronchopulmonary dysplasia, necrotizing enterocolitis, patent ductus arteriosus sepsis and growth between birth and 36 weeks.
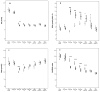
Results: Infants on SMOFlipid® had higher fractions of omega-3 LCPUFA eicosapentaenoic acid (EPA) and slightly higher omega-3 LCPUFA docosahexaenoic acid (DHA) fraction and a decreased arachidonic acid (AA) to DHA ratio from one week after birth up to 32 postmenstrual weeks compared to infants on Clinoleic®. Treatment groups did not differ in morbidities or growth.
Conclusion: Supplementation with SMOFlipid® containing 15% fish oil during parenteral nutrition increased EPA substantially, DHA marginally, reduced AA and decreased AA to DHA ratio. It did not reduce morbidity or affect growth. Since extremely preterm infants accumulate a large deficit of DHA and AA, studies on more prolonged or different levels of DHA and AA supplementation are warranted.
Keywords: Growth; Long-chain polyunsaturated fatty acids; Morbidities; Parenteral nutrition; Preterm.
Copyright © 2017 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
The authors have no potential conflicts of interest relevant to this article to disclose.
Figures
References
-
- Crawford M. Placental delivery of arachidonic and docosahexaenoic acids: implications for the lipid nutrition of preterm infants. Am J Clin Nutr. 2000;71:275S–84S. - PubMed
-
- Haggarty P. Placental regulation of fatty acid delivery and its effect on fetal growth-a review. Placenta. 2002;23:S28–38. - PubMed
-
- Adarme-Vega TC, Thomas-Hall SR, Schenk PM. Towards sustainable sources for omega-3 fatty acids production. Curr Opin Biotechnol. 2014;26:14–8. - PubMed
-
- Uauy R, Mena P, Wegher B, Nieto S, Salem N., Jr Long chain polyunsaturated fatty acid formation in neonates: effect of gestational age and intrauterine growth. Pediatr Res. 2000;47:127–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

