Molecular determinants of beta-adrenergic signaling to voltage-gated K+ channels in the cerebral circulation
- PMID: 29072364
- PMCID: PMC6407422
- DOI: 10.1111/micc.12425
Molecular determinants of beta-adrenergic signaling to voltage-gated K+ channels in the cerebral circulation
Abstract
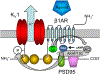
Voltage-gated K+ (Kv ) channels are major determinants of membrane potential in vascular smooth muscle cells (VSMCs) and regulate the diameter of small cerebral arteries and arterioles. However, the intracellular structures that govern the expression and function of vascular Kv channels are poorly understood. Scaffolding proteins including postsynaptic density 95 (PSD95) recently were identified in rat cerebral VSMCs. Primarily characterized in neurons, the PSD95 scaffold has more than 50 known binding partners, and it can mediate macromolecular signaling between cell-surface receptors and ion channels. In cerebral arteries, Shaker-type Kv 1 channels appear to associate with the PSD95 molecular scaffold, and PSD95 is required for the normal expression and vasodilator influence of members of this K+ channel gene family. Furthermore, recent findings suggest that the β1-subtype adrenergic receptor is expressed in cerebral VSMCs and forms a functional vasodilator complex with Kv 1 channels on the PSD95 scaffold. Activation of β1-subtype adrenergic receptors in VSMCs enables protein kinase A-dependent phosphorylation and opening of Kv 1 channels in the PSD95 complex; the subsequent K+ efflux mediates membrane hyperpolarization and vasodilation of small cerebral arteries. Early evidence from other studies suggests that other families of Kv channels and scaffolding proteins are expressed in VSMCs. Future investigations into these macromolecular complexes that modulate the expression and function of Kv channels may reveal unknown signaling cascades that regulate VSMC excitability and provide novel targets for ion channel-based medications to optimize vascular tone.
Keywords: beta adrenergic receptors; potassium channels; scaffolding proteins; smooth muscle.
© 2017 John Wiley & Sons Ltd.
Figures

Similar articles
-
Postsynaptic density-95 scaffolding of Shaker-type K⁺ channels in smooth muscle cells regulates the diameter of cerebral arteries.J Physiol. 2011 Nov 1;589(Pt 21):5143-52. doi: 10.1113/jphysiol.2011.213843. Epub 2011 Sep 12. J Physiol. 2011. PMID: 21911612 Free PMC article.
-
Beta1-adrenergic receptor-mediated dilation of rat cerebral artery requires Shaker-type KV1 channels on PSD95 scaffold.J Cereb Blood Flow Metab. 2015 Sep;35(9):1537-46. doi: 10.1038/jcbfm.2015.91. Epub 2015 May 13. J Cereb Blood Flow Metab. 2015. PMID: 25966954 Free PMC article.
-
Protein kinase A-phosphorylated KV1 channels in PSD95 signaling complex contribute to the resting membrane potential and diameter of cerebral arteries.Circ Res. 2014 Apr 11;114(8):1258-67. doi: 10.1161/CIRCRESAHA.114.303167. Epub 2014 Feb 28. Circ Res. 2014. PMID: 24585759 Free PMC article.
-
KV channels and the regulation of vascular smooth muscle tone.Microcirculation. 2018 Jan;25(1):10.1111/micc.12421. doi: 10.1111/micc.12421. Microcirculation. 2018. PMID: 28985443 Free PMC article. Review.
-
Molecular determinants of voltage-gated potassium currents in vascular smooth muscle.Cell Biochem Biophys. 2005;42(2):167-95. doi: 10.1385/CBB:42:2:167. Cell Biochem Biophys. 2005. PMID: 15858231 Review.
Cited by
-
Ion channel molecular complexes in vascular smooth muscle.Front Physiol. 2022 Aug 26;13:999369. doi: 10.3389/fphys.2022.999369. eCollection 2022. Front Physiol. 2022. PMID: 36091375 Free PMC article. Review.
-
Hydrogen Sulfide-Induced Vasodilation: The Involvement of Vascular Potassium Channels.Front Pharmacol. 2022 Jun 1;13:911704. doi: 10.3389/fphar.2022.911704. eCollection 2022. Front Pharmacol. 2022. PMID: 35721210 Free PMC article. Review.
References
-
- Faraci FM, Baumbach GL, Heistad DD. Myogenic mechanisms in the cerebral circulation. J Hypertens Suppl 1989; 7: S61–4; discussion S65. - PubMed
-
- Knot HJ and Nelson MT. Regulation of membrane potential and diameter by voltage-dependent K+ channels in rabbit myogenic cerebral arteries. Am J Physiol 1995; 269: H348–55. - PubMed
-
- Robertson BE and Nelson MT. Aminopyridine inhibition and voltage dependence of K+ currents in smooth muscle cells from cerebral arteries. Am J Physiol 1994; 267: C1589–97. - PubMed
-
- Kitazono T, Faraci FM, Taguchi H, Heistad DD. Role of potassium channels in cerebral blood vessels. Stroke 1995; 26: 1713–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

