Prostate Cancer Detection with a Tactile Resonance Sensor-Measurement Considerations and Clinical Setup
- PMID: 29072592
- PMCID: PMC5713103
- DOI: 10.3390/s17112453
Prostate Cancer Detection with a Tactile Resonance Sensor-Measurement Considerations and Clinical Setup
Abstract
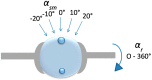
Tumors in the human prostate are usually stiffer compared to surrounding non-malignant glandular tissue, and tactile resonance sensors measuring stiffness can be used to detect prostate cancer. To explore this further, we used a tactile resonance sensor system combined with a rotatable sample holder where whole surgically removed prostates could be attached to detect tumors on, and beneath, the surface ex vivo. Model studies on tissue phantoms made of silicone and porcine tissue were performed. Finally, two resected human prostate glands were studied. Embedded stiff silicone inclusions placed 4 mm under the surface could be detected in both the silicone and biological tissue models, with a sensor indentation of 0.6 mm. Areas with different amounts of prostate cancer (PCa) could be distinguished from normal tissue (p < 0.05), when the tumor was located in the anterior part, whereas small tumors located in the dorsal aspect were undetected. The study indicates that PCa may be detected in a whole resected prostate with an uneven surface and through its capsule. This is promising for the development of a clinically useful instrument to detect prostate cancer during surgery.
Keywords: piezoelectric sensor; prostate cancer; resonance sensor; tactile sensor; tissue stiffness.
Conflict of interest statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures












References
-
- Omata S., Terunuma Y. New tactile sensor like the human hand and its applications. Sens. Actuators A Phys. 1992;35:9–15. doi: 10.1016/0924-4247(92)87002-X. - DOI
-
- Sasai S., Zhen Y.-X., Suetake T., Tanita Y., Omata S., Tagami H. Palpation of the skin with a robot finger: An attempt to measure skin stiffness with a probe loaded with a newly developed tactile vibration sensor and displacement sensor. Skin Res. Technol. 1999;5:237–246. doi: 10.1111/j.1600-0846.1999.tb00136.x. - DOI
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

