Long-term patient-reported outcomes from an open-label safety and efficacy study of bosutinib in Philadelphia chromosome-positive chronic myeloid leukemia patients resistant or intolerant to prior therapy
- PMID: 29072772
- PMCID: PMC5813200
- DOI: 10.1002/cncr.31082
Long-term patient-reported outcomes from an open-label safety and efficacy study of bosutinib in Philadelphia chromosome-positive chronic myeloid leukemia patients resistant or intolerant to prior therapy
Abstract
Background: Health-related quality of life (HRQOL) in patients with chronic-phase chronic myeloid leukemia (CML) is important because of the requirement for long-term treatment. This study assessed HRQOL in bosutinib-treated patients with Philadelphia chromosome-positive CML and resistance or intolerance to 1 (chronic-phase second-line [CP2L]) or more (chronic-phase third-line [CP3L]) tyrosine kinase inhibitors who had 264 weeks or more of follow-up (ClinicalTrials.gov identifier NCT00261846).
Methods: Patient-reported HRQOL was assessed with the EuroQol 5-Dimensions Questionnaire (EQ-5D) and the Functional Assessment of Cancer Therapy-Leukemia (FACT-Leu).
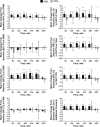
Results: In total, 284 and 119 patients composed the CP2L and CP3L cohorts, respectively. At treatment completion, more than 50% of the patients in the CP2L and CP3L cohorts completed the EQ-5D and FACT-Leu assessments. The EQ-5D and EQ-5D visual analog scale scores were stable in both cohorts throughout treatment. The mean FACT-Leu scores were generally stable over time but were lower in magnitude in the CP3L cohort versus the CP2L cohort. The FACT-Leu scale scores of a subset of patients with chronic diarrhea (CP2L, n = 101; CP3L, n = 30) were similar to the scores of the larger cohorts. Minimally important differences (MIDs) from baseline for the FACT-Leu scale scores were observed for the following: emotional well-being (EWB), Functional Assessment of Cancer Therapy-General (FACT-G) Total, FACT-Leu Total, and Functional Assessment of Cancer Therapy Trial Outcome Index (FACT-TOI) in the CP2L cohort and FACT-Leu Total in the CP3L cohort. Among patients with chronic diarrhea, MIDs were observed for EWB, FACT-G Total, FACT-Leu Total, and FACT-TOI in the CP2L cohort and for EWB, FACT-G Total, and FACT-Leu Total in the CP3L cohort.
Conclusions: HRQOL was maintained with long-term bosutinib treatment for patients with CP2L and CP3L CML. Cancer 2018;124:587-95. © 2017 The Authors. Cancer published by Wiley Periodicals, Inc. on behalf of American Cancer Society. This is an open access article under the terms of the Creative Commons Attribution-NonCommercial License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited and is not used for commercial purposes.
Keywords: bosutinib; chronic myeloid leukemia; patient-reported outcomes; quality of life; tyrosine kinase inhibitors.
© 2017 The Authors. Cancer published by Wiley Periodicals, Inc. on behalf of American Cancer Society.
Figures


References
-
- O'Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low‐dose cytarabine for newly diagnosed chronic‐phase chronic myeloid leukemia. N Engl J Med. 2003;348:994‐1004. - PubMed
-
- Druker BJ, Guilhot F, O'Brien SG, et al. Five‐year follow‐up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408‐2417. - PubMed
-
- Larson RA, Hochhaus A, Hughes TP, et al. Nilotinib vs imatinib in patients with newly diagnosed Philadelphia chromosome–positive chronic myeloid leukemia in chronic phase: ENESTnd 3‐year follow‐up. Leukemia. 2012;26:2197‐2203. - PubMed
-
- Hochhaus A, Kantarjian HM, Baccarani M, et al. Dasatinib induces notable hematologic and cytogenetic responses in chronic‐phase chronic myeloid leukemia after failure of imatinib therapy. Blood. 2007;109:2303‐2309. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

