Distinct ice patterns on solid surfaces with various wettabilities
- PMID: 29073045
- PMCID: PMC5664549
- DOI: 10.1073/pnas.1712829114
Distinct ice patterns on solid surfaces with various wettabilities
Abstract
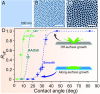
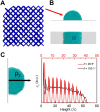
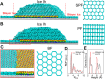
No relationship has been established between surface wettability and ice growth patterns, although ice often forms on top of solid surfaces. Here, we report experimental observations obtained using a process specially designed to avoid the influence of nucleation and describe the wettability-dependent ice morphology on solid surfaces under atmospheric conditions and the discovery of two growth modes of ice crystals: along-surface and off-surface growth modes. Using atomistic molecular dynamics simulation analysis, we show that these distinct ice growth phenomena are attributable to the presence (or absence) of bilayer ice on solid surfaces with different wettability; that is, the formation of bilayer ice on hydrophilic surface can dictate the along-surface growth mode due to the structural match between the bilayer hexagonal ice and the basal face of hexagonal ice (ice Ih), thereby promoting rapid growth of nonbasal faces along the hydrophilic surface. The dramatically different growth patterns of ice on solid surfaces are of crucial relevance to ice repellency surfaces.
Keywords: antiicing; ice crystal; ice growth; molecular dynamics simulation; surface wettability.
Published under the PNAS license.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











References
-
- Atkinson JD, et al. The importance of feldspar for ice nucleation by mineral dust in mixed-phase clouds. Nature. 2013;498:355–358. - PubMed
-
- Ball P, Stillinger FH. H2O: A biography of water. Nature. 1999;401:850–850.
-
- Murray BJ, Knopf DA, Bertram AK. The formation of cubic ice under conditions relevant to Earth’s atmosphere. Nature. 2005;434:202–205. - PubMed
-
- Abbatt JPD, et al. Solid ammonium sulfate aerosols as ice nuclei: A pathway for cirrus cloud formation. Science. 2006;313:1770–1773. - PubMed
-
- Doxey AC, Yaish MW, Griffith M, McConkey BJ. Ordered surface carbons distinguish antifreeze proteins and their ice-binding regions. Nat Biotechnol. 2006;24:852–855. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

