FGF4 retrogene on CFA12 is responsible for chondrodystrophy and intervertebral disc disease in dogs
- PMID: 29073074
- PMCID: PMC5664524
- DOI: 10.1073/pnas.1709082114
FGF4 retrogene on CFA12 is responsible for chondrodystrophy and intervertebral disc disease in dogs
Abstract
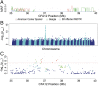
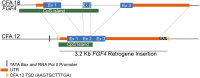
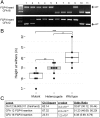
Chondrodystrophy in dogs is defined by dysplastic, shortened long bones and premature degeneration and calcification of intervertebral discs. Independent genome-wide association analyses for skeletal dysplasia (short limbs) within a single breed (PBonferroni = 0.01) and intervertebral disc disease (IVDD) across breeds (PBonferroni = 4.0 × 10-10) both identified a significant association to the same region on CFA12. Whole genome sequencing identified a highly expressed FGF4 retrogene within this shared region. The FGF4 retrogene segregated with limb length and had an odds ratio of 51.23 (95% CI = 46.69, 56.20) for IVDD. Long bone length in dogs is a unique example of multiple disease-causing retrocopies of the same parental gene in a mammalian species. FGF signaling abnormalities have been associated with skeletal dysplasia in humans, and our findings present opportunities for both selective elimination of a medically and financially devastating disease in dogs and further understanding of the ever-growing complexity of retrogene biology.
Keywords: GWAS; chondrodysplasia; dysplasia; genetic; inherited.
Copyright © 2017 the Author(s). Published by PNAS.
Conflict of interest statement
Conflict of interest statement: The University of California, Davis, has filed a provisional patent entitled: “Methods of Diagnosing Intervertebral Disc Disease and Chondrodystrophy in Canines,” on May 30, 2017.
Figures


References
-
- Hansen H-J. A pathologic-anatomical study on disc degeneration in dog: With special reference to the so-called enchondrosis intervertebralis. Acta Orthop Scand. 1952;23(Suppl 11):1–130. - PubMed
-
- Hansen H-J. A pathologic-anatomical interpretation of disc degeneration in dogs. Acta Orthop Scand. 1951;20:280–293. - PubMed
-
- Braund KG, Ghosh P, Taylor TK, Larsen LH. Morphological studies of the canine intervertebral disc. The assignment of the beagle to the achondroplastic classification. Res Vet Sci. 1975;19:167–172. - PubMed
-
- Bray JP, Burbidge HM. The canine intervertebral disk. Part two: Degenerative changes—Nonchondrodystrophoid versus chondrodystrophoid disks. J Am Anim Hosp Assoc. 1998;34:135–144. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

