TREM2 deficiency attenuates neuroinflammation and protects against neurodegeneration in a mouse model of tauopathy
- PMID: 29073081
- PMCID: PMC5663386
- DOI: 10.1073/pnas.1710311114
TREM2 deficiency attenuates neuroinflammation and protects against neurodegeneration in a mouse model of tauopathy
Abstract
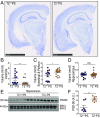
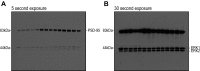
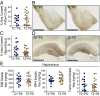
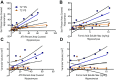
Variants in the gene encoding the triggering receptor expressed on myeloid cells 2 (TREM2) were recently found to increase the risk for developing Alzheimer's disease (AD). In the brain, TREM2 is predominately expressed on microglia, and its association with AD adds to increasing evidence implicating a role for the innate immune system in AD initiation and progression. Thus far, studies have found TREM2 is protective in the response to amyloid pathology while variants leading to a loss of TREM2 function impair microglial signaling and are deleterious. However, the potential role of TREM2 in the context of tau pathology has not yet been characterized. In this study, we crossed Trem2+/+ (T2+/+) and Trem2-/- (T2-/-) mice to the PS19 human tau transgenic line (PS) to investigate whether loss of TREM2 function affected tau pathology, the microglial response to tau pathology, or neurodegeneration. Strikingly, by 9 mo of age, T2-/-PS mice exhibited significantly less brain atrophy as quantified by ventricular enlargement and preserved cortical volume in the entorhinal and piriform regions compared with T2+/+PS mice. However, no TREM2-dependent differences were observed for phosphorylated tau staining or insoluble tau levels. Rather, T2-/-PS mice exhibited significantly reduced microgliosis in the hippocampus and piriform cortex compared with T2+/+PS mice. Gene expression analyses and immunostaining revealed microglial activation was significantly attenuated in T2-/-PS mice, and there were lower levels of inflammatory cytokines and astrogliosis. These unexpected findings suggest that impairing microglial TREM2 signaling reduces neuroinflammation and is protective against neurodegeneration in the setting of pure tauopathy.
Keywords: Alzheimer’s disease; TREM2; neurodegeneration; neuroinflammation; tau.
Copyright © 2017 the Author(s). Published by PNAS.
Conflict of interest statement
Conflict of interest statement: C.E.G.L. and D.M.H. are listed as inventors on a patent licensed by Washington University to C2N Diagnostics on the therapeutic use of anti-tau antibodies. D.M.H. cofounded and is on the scientific advisory board of C2N Diagnostics, LLC. C2N Diagnostics, LLC, has licensed certain anti-tau antibodies to AbbVie for therapeutic development. D.M.H. is on the scientific advisory board of Proclara Biosciences and consults for Genentech, Eli Lilly, AbbVie, GlaxoSmithKline, and Denali. J.D.U., M.B.F., F.R.S., L.J.K., J.R.S., G.O.R., E.A., and M.C. declare no competing financial interests.
Figures




References
-
- Itagaki S, McGeer PL, Akiyama H, Zhu S, Selkoe D. Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J Neuroimmunol. 1989;24:173–182. - PubMed
-
- Sheffield LG, Marquis JG, Berman NE. Regional distribution of cortical microglia parallels that of neurofibrillary tangles in Alzheimer’s disease. Neurosci Lett. 2000;285:165–168. - PubMed
-
- Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease—a double-edged sword. Neuron. 2002;35:419–432. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

