Spatial organization of a model 15-member human gut microbiota established in gnotobiotic mice
- PMID: 29073107
- PMCID: PMC5664539
- DOI: 10.1073/pnas.1711596114
Spatial organization of a model 15-member human gut microbiota established in gnotobiotic mice
Abstract
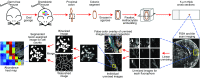
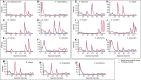
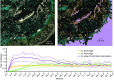
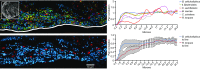
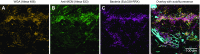
Knowledge of the spatial organization of the gut microbiota is important for understanding the physical and molecular interactions among its members. These interactions are thought to influence microbial succession, community stability, syntrophic relationships, and resiliency in the face of perturbations. The complexity and dynamism of the gut microbiota pose considerable challenges for quantitative analysis of its spatial organization. Here, we illustrate an approach for addressing this challenge, using (i) a model, defined 15-member consortium of phylogenetically diverse, sequenced human gut bacterial strains introduced into adult gnotobiotic mice fed a polysaccharide-rich diet, and (ii) in situ hybridization and spectral imaging analysis methods that allow simultaneous detection of multiple bacterial strains at multiple spatial scales. Differences in the binding affinities of strains for substrates such as mucus or food particles, combined with more rapid replication in a preferred microhabitat, could, in principle, lead to localized clonally expanded aggregates composed of one or a few taxa. However, our results reveal a colonic community that is mixed at micrometer scales, with distinct spatial distributions of some taxa relative to one another, notably at the border between the mucosa and the lumen. Our data suggest that lumen and mucosa in the proximal colon should be conceptualized not as stratified compartments but as components of an incompletely mixed bioreactor. Employing the experimental approaches described should allow direct tests of whether and how specified host and microbial factors influence the nature and functional contributions of "microscale" mixing to the dynamic operations of the microbiota in health and disease.
Keywords: bacterial–bacterial interactions; community biogeography; gut microbial ecology; microbiome function; multiplex fluorescence imaging.
Published under the PNAS license.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Kolenbrander PE, Palmer RJ, Jr, Periasamy S, Jakubovics NS. Oral multispecies biofilm development and the key role of cell-cell distance. Nat Rev Microbiol. 2010;8:471–480. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

