Prolonged immune alteration following resolution of acute inflammation in humans
- PMID: 29073216
- PMCID: PMC5658111
- DOI: 10.1371/journal.pone.0186964
Prolonged immune alteration following resolution of acute inflammation in humans
Abstract
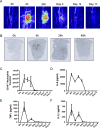
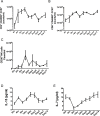
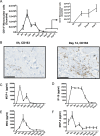
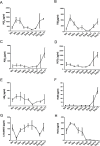
Acute inflammation is an immediate response to infection and injury characterised by the influx of granulocytes followed by phagocytosing mononuclear phagocytes. Provided the antigen is cleared and the immune system of the host is fully functional, the acute inflammatory response will resolve. Until now it is considered that resolution then leads back to homeostasis, the physiological state tissues experienced before inflammation occurred. Using a human model of acute inflammation driven by intradermal UV killed Escherichia coli, we found that bacteria and granulocyte clearance as well as pro-inflammatory cytokine catabolism occurred by 72h. However, following a lag phase of about 4 days there was an increase in numbers of memory T cells and CD163+ macrophage at the post-resolution site up to day 17 as well as increased biosynthesis of cyclooxygenase-derived prostanoids and DHA-derived D series resolvins. Inhibiting post-resolution prostanoids using naproxen showed that numbers of tissue memory CD4 cells were under the endogenous control of PGE2, which exerts its suppressive effects on T cell proliferation via the EP4 receptor. In addition, we re-challenged the post-resolution site with a second injection of E. coli, which when compared to saline controls resulted in primarily a macrophage-driven response with comparatively fewer PMNs; the macrophage-dominated response was reversed by cyclooxygenase inhibition. Re-challenge experiments were also carried out in mice where we obtained similar results as in humans. Therefore, we report that acute inflammatory responses in both humans and rodents do not revert back to homeostasis, but trigger a hitherto unappreciated sequence of immunological events that dictate subsequent immune response to infection.
Conflict of interest statement
Figures
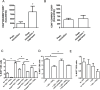
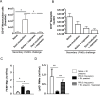
Comment in
-
Editorial Note: Prolonged immune alteration following resolution of acute inflammation in humans.PLoS One. 2025 Mar 31;20(3):e0321981. doi: 10.1371/journal.pone.0321981. eCollection 2025. PLoS One. 2025. PMID: 40163472 Free PMC article. No abstract available.
References
-
- Segal A, Peters T. Characterisation of the enzyme defect in chronic granulomatous disease. Lancet. 1976;1: 1363–5. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

