MiRNA-124 is a link between measles virus persistent infection and cell division of human neuroblastoma cells
- PMID: 29073265
- PMCID: PMC5658143
- DOI: 10.1371/journal.pone.0187077
MiRNA-124 is a link between measles virus persistent infection and cell division of human neuroblastoma cells
Abstract
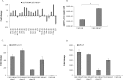
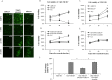
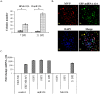
Measles virus (MV) infects a variety of lymphoid and non-lymphoid peripheral organs. However, in rare cases, the virus can persistently infect cells within the central nervous system. Although some of the factors that allow MV to persist are known, the contribution of host cell-encoded microRNAs (miRNA) have not been described. MiRNAs are a class of noncoding RNAs transcribed from genomes of all multicellular organisms and some viruses, which regulate gene expression in a sequence-specific manner. We have studied the contribution of host cell-encoded miRNAs to the establishment of MV persistent infection in human neuroblastoma cells. Persistent MV infection was accompanied by differences in the expression profile and levels of several host cell-encoded microRNAs as compared to uninfected cells. MV persistence infection of a human neuroblastoma cell line (UKF-NB-MV), exhibit high miRNA-124 expression, and reduced expression of cyclin dependent kinase 6 (CDK6), a known target of miRNA-124, resulting in slower cell division but not cell death. By contrast, acute MV infection of UKF-NB cells did not result in increased miRNA-124 levels or CDK6 reduction. Ectopic overexpression of miRNA-124 affected cell viability only in UKF-NB-MV cells, causing cell death; implying that miRNA-124 over expression can sensitize cells to death only in the presence of MV persistent infection. To determine if miRNA-124 directly contributes to the establishment of MV persistence, UKF-NB cells overexpressing miRNA-124 were acutely infected, resulting in establishment of persistently infected colonies. We propose that miRNA-124 triggers a CDK6-dependent decrease in cell proliferation, which facilitates the establishment of MV persistence in neuroblastoma cells. To our knowledge, this is the first report to describe the role of a specific miRNA in MV persistence.
Conflict of interest statement
Figures




Similar articles
-
Persistent human cytomegalovirus infection induces drug resistance and alteration of programmed cell death in human neuroblastoma cells.Cancer Res. 1998 Jan 15;58(2):367-72. Cancer Res. 1998. PMID: 9443419
-
microRNA-449a functions as a tumor suppressor in neuroblastoma through inducing cell differentiation and cell cycle arrest.RNA Biol. 2015;12(5):538-54. doi: 10.1080/15476286.2015.1023495. RNA Biol. 2015. PMID: 25760387 Free PMC article.
-
Chemoresistance induces enhanced adhesion and transendothelial penetration of neuroblastoma cells by down-regulating NCAM surface expression.BMC Cancer. 2006 Dec 21;6:294. doi: 10.1186/1471-2407-6-294. BMC Cancer. 2006. PMID: 17181871 Free PMC article.
-
Deciphering the Role of MicroRNAs in Neuroblastoma.Molecules. 2021 Dec 24;27(1):99. doi: 10.3390/molecules27010099. Molecules. 2021. PMID: 35011335 Free PMC article. Review.
-
N-myc and noncoding RNAs in neuroblastoma.Mol Cancer Res. 2012 Oct;10(10):1243-53. doi: 10.1158/1541-7786.MCR-12-0244. Epub 2012 Aug 30. Mol Cancer Res. 2012. PMID: 22936790 Review.
Cited by
-
Measles Virus Persistent Infection of Human Induced Pluripotent Stem Cells.Cell Reprogram. 2018 Feb;20(1):17-26. doi: 10.1089/cell.2017.0034. Cell Reprogram. 2018. PMID: 29412740 Free PMC article.
-
Overview of host-directed antiviral targets for future research and drug development.Acta Pharm Sin B. 2025 Apr;15(4):1723-1751. doi: 10.1016/j.apsb.2025.03.011. Epub 2025 Mar 8. Acta Pharm Sin B. 2025. PMID: 40486850 Free PMC article. Review.
-
Small Non-coding RNA Expression Following Respiratory Syncytial Virus or Measles Virus Infection of Neuronal Cells.Front Microbiol. 2021 Sep 3;12:671852. doi: 10.3389/fmicb.2021.671852. eCollection 2021. Front Microbiol. 2021. PMID: 34539595 Free PMC article.
-
High-Throughput Fluorescence-Based Screen Identifies the Neuronal MicroRNA miR-124 as a Positive Regulator of Alphavirus Infection.J Virol. 2020 Apr 16;94(9):e02145-19. doi: 10.1128/JVI.02145-19. Print 2020 Apr 16. J Virol. 2020. PMID: 32102877 Free PMC article.
-
Nuphar lutea Extracts Exhibit Anti-Viral Activity against the Measles Virus.Molecules. 2020 Apr 3;25(7):1657. doi: 10.3390/molecules25071657. Molecules. 2020. PMID: 32260270 Free PMC article.
References
-
- Griffin DE, Lin WH, Pan CH. Measles virus, immune control, and persistence. FEMS Microbiol Rev. 2012;36(3):649–62. doi: 10.1111/j.1574-6976.2012.00330.x . - DOI - PMC - PubMed
-
- Moss WJ, Griffin DE. Measles Lancet. 379 England: 2012 Elsevier Ltd; 2012. p. 153–64. doi: 10.1016/S0140-6736(10)62352-5 - DOI - PubMed
-
- Lin WH, Kouyos RD, Adams RJ, Grenfell BT, Griffin DE. Prolonged persistence of measles virus RNA is characteristic of primary infection dynamics. Proc Natl Acad Sci U S A. 2012;109(37):14989–94. doi: 10.1073/pnas.1211138109 . - DOI - PMC - PubMed
-
- Schneider-Schaulies J, Niewiesk S, Schneider-Schaulies S, ter Meulen V. Measles virus in the CNS: the role of viral and host factors for the establishment and maintenance of a persistent infection. J Neurovirol. 1999;5(6):613–22. Epub 1999/12/22. . - PubMed
-
- Rall GF. Measles virus 1998–2002: progress and controversy. Annu Rev Microbiol. 2003;57:343–67. Epub 2003/10/07. doi: 10.1146/annurev.micro.57.030502.090843 . - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

