Accumulation of Antigen-Driven Lymphoproliferations in Complement Receptor 2/CD21-/low B Cells From Patients With Sjögren's Syndrome
- PMID: 29073352
- PMCID: PMC5788702
- DOI: 10.1002/art.40352
Accumulation of Antigen-Driven Lymphoproliferations in Complement Receptor 2/CD21-/low B Cells From Patients With Sjögren's Syndrome
Abstract
Objective: Patients with Sjögren's syndrome (SS) are prone to develop malignant lymphomas, and a correlation has been established between the lymphoproliferations occurring in these disorders and the presence in patients' blood of an unusual B cell population that down-regulates complement receptor 2/CD21. This study was undertaken to identify the B cell compartment from which these lymphoproliferations emerge and determine the mechanisms that promote clonal B cell expansion in patients with SS.
Methods: The reactivity of antibodies expressed by CD19+CD10-CD27-IgM+CD21-/low cells isolated from the blood of patients with SS was tested using a polymerase chain reaction-based approach that allows us to clone and express, in vitro, recombinant antibodies produced by single B cells.
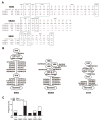
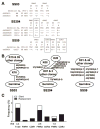
Results: Clonal expansions were identified in CD21-/low B cells isolated from the peripheral blood of 3 patients with SS. These lymphoproliferations expressed B cell receptors (BCRs) that displayed somatic hypermutation lineage trees characteristic of a strong selection by antigens; one of these antigens was identified as a ribosomal self antigen. When the mutated BCR sequences expressed by the expanded CD21-/low B cell clones from patients with SS were reverted in vitro to their germline counterparts, one clone remained autoreactive.
Conclusion: Clonal lymphoproliferations in patients with SS preferentially accumulate in the autoreactive CD21-/low B cell compartment often expanded in these subjects, and recognition of self antigens may drive the clonal B cell expansion while further refining BCR self-reactivity.
© 2017, American College of Rheumatology.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Comment in
-
Rheumatoid Factor Reactivity of Expanded CD21-/low B Cells in Patients With Sjögren's Syndrome: Comment on the Article by Glauzy et al.Arthritis Rheumatol. 2019 Jan;71(1):169-170. doi: 10.1002/art.40756. Epub 2018 Nov 21. Arthritis Rheumatol. 2019. PMID: 30303268 No abstract available.
Similar articles
-
Expansion of autoreactive unresponsive CD21-/low B cells in Sjögren's syndrome-associated lymphoproliferation.Arthritis Rheum. 2013 Apr;65(4):1085-96. doi: 10.1002/art.37828. Arthritis Rheum. 2013. PMID: 23279883 Free PMC article.
-
Defective Early B Cell Tolerance Checkpoints in Sjögren's Syndrome Patients.Arthritis Rheumatol. 2017 Nov;69(11):2203-2208. doi: 10.1002/art.40215. Epub 2017 Oct 12. Arthritis Rheumatol. 2017. PMID: 28704602 Free PMC article.
-
Salivary gland B cell lymphoproliferative disorders in Sjögren's syndrome present a restricted use of antigen receptor gene segments similar to those used by hepatitis C virus-associated non-Hodgkins's lymphomas.Eur J Immunol. 2002 Mar;32(3):903-10. doi: 10.1002/1521-4141(200203)32:3<903::AID-IMMU903>3.0.CO;2-D. Eur J Immunol. 2002. PMID: 11870635
-
Preliminary classification of nonmalignant B cell proliferation in Sjögren's syndrome: perspectives on pathobiology and treatment based on an integrated clinico-pathologic and molecular study approach.Blood Cells Mol Dis. 2001 Jul-Aug;27(4):757-66. doi: 10.1006/bcmd.2001.0446. Blood Cells Mol Dis. 2001. PMID: 11778660 Review.
-
B-cell lymphoproliferation in primary Sjögren's syndrome.Clin Exp Rheumatol. 1996 Jan-Feb;14 Suppl 14:S65-70. Clin Exp Rheumatol. 1996. PMID: 8722203 Review.
Cited by
-
CD11c+ B Cells Participate in the Pathogenesis of Graves' Disease by Secreting Thyroid Autoantibodies and Cytokines.Front Immunol. 2022 Mar 21;13:836347. doi: 10.3389/fimmu.2022.836347. eCollection 2022. Front Immunol. 2022. PMID: 35386700 Free PMC article.
-
Targeted Therapy for Primary Sjögren's Syndrome: Where are We Now?BioDrugs. 2021 Nov;35(6):593-610. doi: 10.1007/s40259-021-00505-7. Epub 2021 Nov 3. BioDrugs. 2021. PMID: 34731460 Review.
-
Impaired B-cell tolerance checkpoints promote the development of autoimmune diseases and pathogenic autoantibodies.Immunol Rev. 2019 Nov;292(1):90-101. doi: 10.1111/imr.12821. Epub 2019 Nov 12. Immunol Rev. 2019. PMID: 31721234 Free PMC article. Review.
-
Epithelial-immune cell interplay in primary Sjögren syndrome salivary gland pathogenesis.Nat Rev Rheumatol. 2021 Jun;17(6):333-348. doi: 10.1038/s41584-021-00605-2. Epub 2021 Apr 28. Nat Rev Rheumatol. 2021. PMID: 33911236 Free PMC article. Review.
-
High-Throughput Detection of Autoantigen-Specific B Cells Among Distinct Functional Subsets in Autoimmune Donors.Front Immunol. 2021 May 24;12:685718. doi: 10.3389/fimmu.2021.685718. eCollection 2021. Front Immunol. 2021. PMID: 34234784 Free PMC article.
References
-
- Ioannidis JPA, Vassiliou VA, Moutsopoulos HM. Long-term risk of mortality and lymphoproliferative disease and predictive classification of primary Sjögren’s syndrome. Arthritis Rheum. 2002;46(3):741–747. - PubMed
-
- Wardemann H, Yurasov S, Schaefer A, et al. Predominant autoantibody production by early human B cell precursors. Science. 2003;301(5638):1374–1377. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

